Figures & data
Figure 1 Study design.
Abbreviations: R, randomization; pMDI, pressurized metered-dose inhaler.
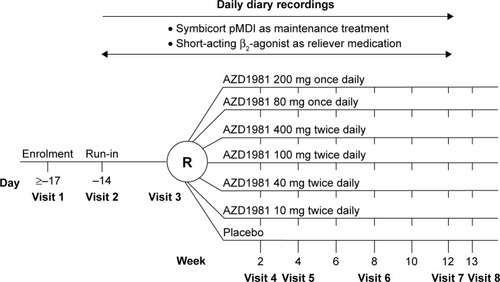
Figure 2 Pattern of AZD1981 tablet formulation dispensed for the different treatment arms.
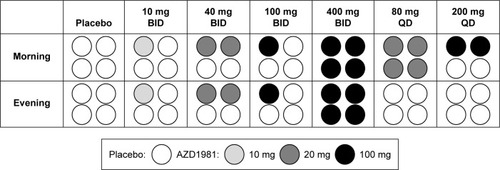
Table 1 Patient demographics and baseline characteristicsTable Footnote*
Figure 3 Patient study flow.
Abbreviations: BID, twice daily; QD, once daily; GCP, Good Clinical Practice.
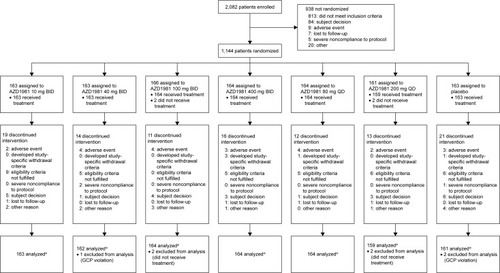
Figure 4 Mean change from baseline.
Abbreviations: BID, twice daily; QD, once daily; GCP, Good Clinical Practice.
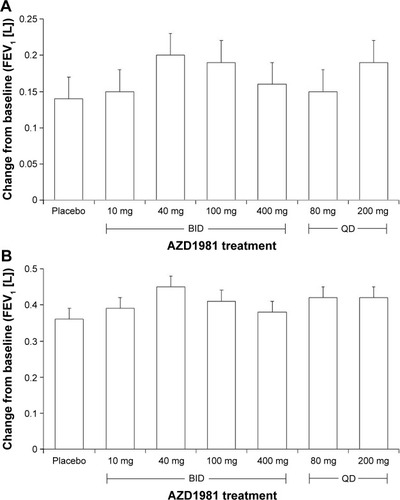
Figure 5 Mean change from baseline in the Asthma Control Questionnaire (ACQ)-5 score.
Abbreviations: BID, twice daily; QD, once daily; GCP, Good Clinical Practice.
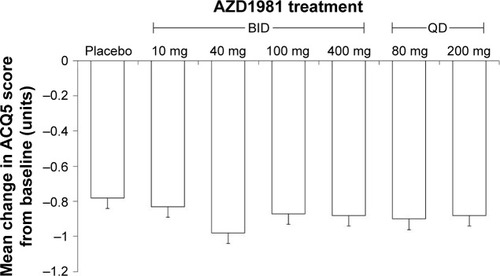
Table 2 Mean change from baseline in morning and evening and PEF and FEV1 measured by the patient at home over 12 weeks of treatmentTable Footnote*
Table 3 Cox regression analysis of time to treatment failureTable Footnote*
Table 4 Summary of adverse events (AEs)Table Footnote*
Table S1 Institutional review boards and ethics committees by country
