Figures & data
Figure 1 Morphology and immunofluorescence in RCECs.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; RCECs, retinal capillary endothelial cells; vWf, von Willebrand factor.
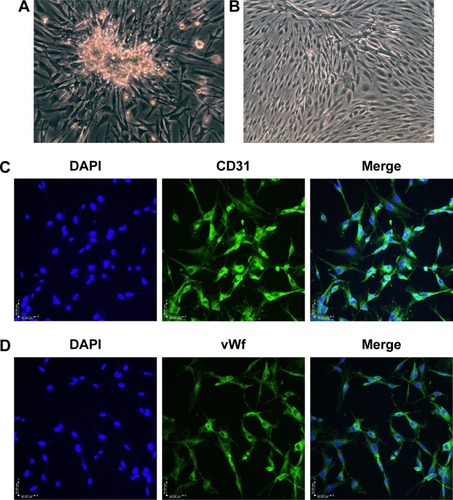
Figure 2 Protection of NR1 from high glucose-induced cytotoxicity.
Abbreviations: LDH, lactate dehydrogenase; NR1, Notoginsenoside R1; RCECs, retinal capillary endothelial cells.
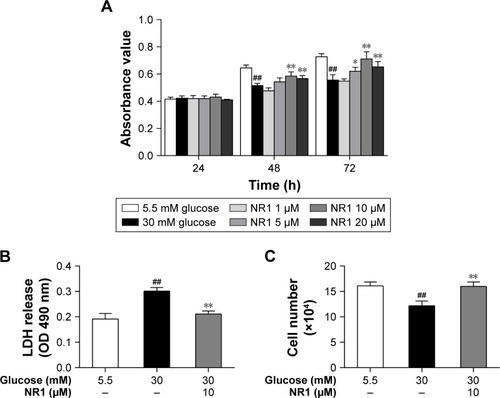
Figure 3 NR1 attenuated high glucose-induced endothelial damage in rat RCECs.
Abbreviations: BF, bright field; mtDNA, mitochondrial DNA; NR1, Notoginsenoside R1; PCR, polymerase chain reaction; PI, propidium iodide; RCECs, retinal capillary endothelial cells.
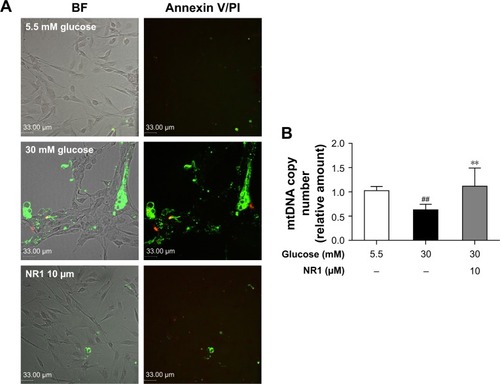
Figure 4 NR1 decreased ROS and 3-NT levels in rat RCECs under high glucose conditions.
Abbreviations: 3-NT, 3-nitrotyrosine; DAPI, 4′,6-diamidino-2-phenylindole; NR1, Notoginsenoside R1; RCECs, retinal capillary endothelial cells; ROS, reactive oxygen species; RFU, relative fluorescence unit.
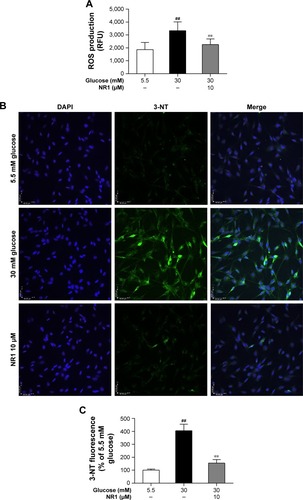
Figure 5 NR1 inhibited NOX and PARP activities, but increased CAT activity under high glucose conditions.
Abbreviations: CAT, catalase; NOX, NADPH oxidase; NR1, Notoginsenoside R1; PARP, poly-ADP (ribose) polymerase; RCECs, retinal capillary endothelial cells; FLU, relative light unit.
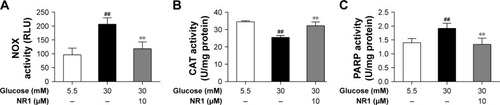
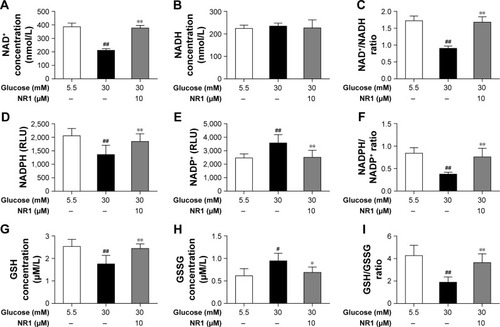
Figure 6 NR1, an intracellular redox modulator, is useful under high glucose conditions when the intracellular redox status is impaired.
Abbreviations: GSH, glutathione; NAD+, nicotinamide adenine dinucleotide+; NR1, Notoginsenoside R1; RCECs, retinal capillary endothelial cells; ROS, reactive oxygen species.