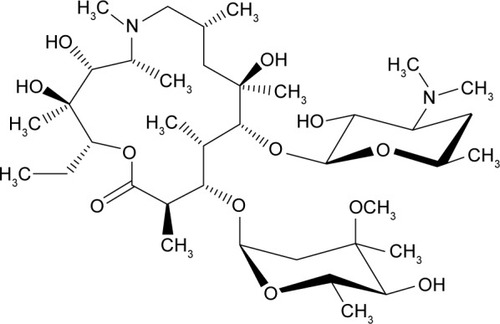
Figures & data
Table 1 Sequential method for the selection of trainee volunteers
Table 2 Preparation of AZNs
Figure 2 (A) Scanning electron micrographs of unprocessed azithromycin, (B) titanium dioxide nanoparticles, and (C) azithromycin nanohybrid.

Figure 3 Langmuir adsorption isotherm of AZ and TNP equilibrium.
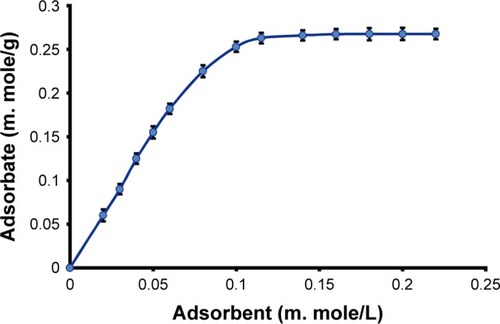
Figure 4 (A) Fourier-transformed infrared spectrum of unprocessed azithromycin, (B) azithromycin nanohybrid, and (C) titanium dioxide nanoparticles.
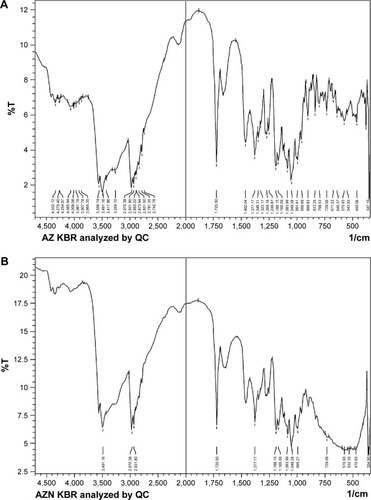
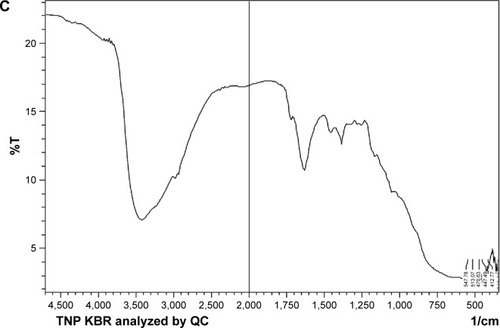
Figure 6 (A) X-ray powder diffraction pattern of TNP, (B) AZ unprocessed, (C) AZN.
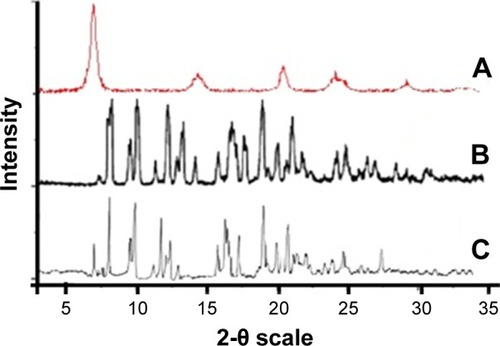
Figure 7 (A) Dissolution rate of formulations (F1–F5) and AZN at pH 7.4. (B) Dissolution rate of formulations (F6–F10) and AZN. (C) Comparative dissolution rate of optimized formulation (F6), marketed drug, AZN, and unprocessed AZ at pH 7.4. (D) Comparative dissolution rate of unprocessed AZ, AZN, standard (marketed) AZN, and optimized formulation (F6) at saliva pH.
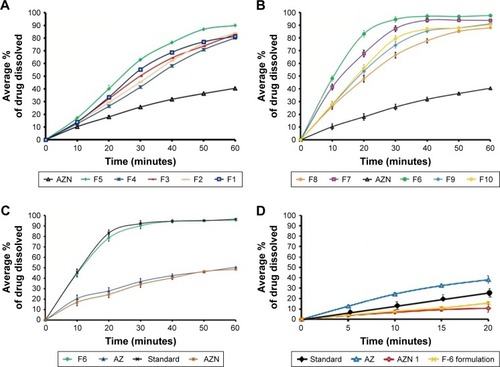
Table 3 Formulation of AZN
Table 4 Physicochemical stability of azithromycin as dry suspension (F6)
Table 5 Panel testing for taste evaluation of various formulations
Table 6 Stability study of optimized formulation (F6) after reconstitution kept at 8°C–15°C