Figures & data
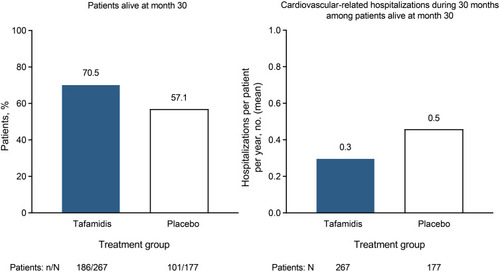
Figure 1 The TTR amyloidogenic cascade. When destabilized by TTR pathogenic variants, the tetrameric protein TTR dissociates into monomers that misfold and misassemble into aggregates, including amyloid fibrils associated with transthyretin amyloidosis.
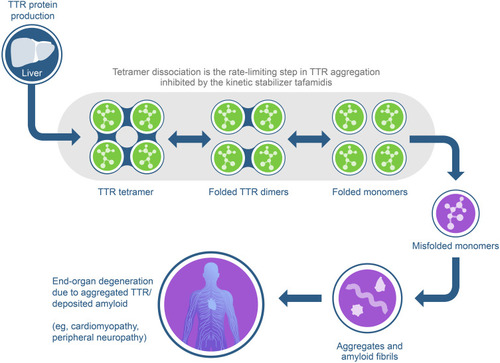
Figure 2 Timeline of the major translational milestones in the development of the TTR kinetic stabilizer tafamidis. Superscript numbers refer to references.
Table 1 Summary of Methods and Results from Phase 2/3 Clinical Studies of Tafamidis in Patients with ATTR-PN and ATTR-CM
Table 2 Adverse Events Reported by Treatment Group in Pivotal Randomized Controlled Trials of Tafamidis in (A) ATTR-PNCitation60 and (B) ATTR-CM (ATTR-ACT)Citation19
Figure 3 (A) Proportion of NIS-LL respondersa at month 18 in the ITTb and EE populations. (B) LS mean (±SE) change from baseline to month 18 in the TQoL score from the Norfolk QoL-DN in the ITTb and EE populations. (C) LS mean (±SE) changes from baseline at months 6, 12, and 18 in the NIS-LL in the ITT population.Citation90,Citation97 aResponse = increase of <2 in the NIS-LL overall score. bProportion of NIS-LL responders and LS mean change from baseline in TQoL score at month 18 in the ITT population were coprimary endpoints of the study.
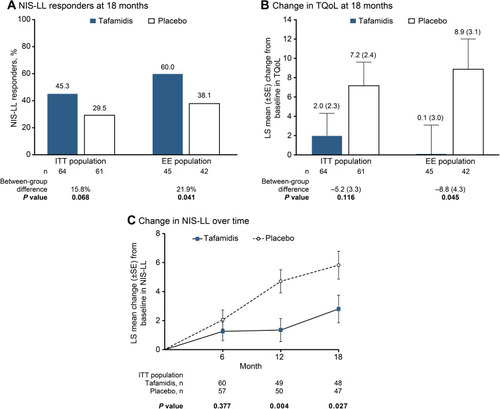
Figure 4 All-cause mortality and frequency of cardiovascular-related hospitalizations (primary efficacy analysis) in patients with ATTR-CM treated with tafamidis versus placebo in ATTR-ACT.Citation19 The combined primary endpoint was hierarchically assessed using the Finkelstein–Schoenfeld method. P < 0.001.