Figures & data
Table 1 Timeline of Events in the Development of Lonapegsomatropin
Figure 1 Timeline of daily GH and long-acting GH product availability.
Figure 2 Schematic overview of lonapegsomatropin.Citation24
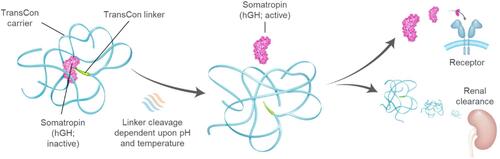
Table 2 Comparison of Growth Responses Between Long-Acting and Daily Growth Hormones in Pediatric Growth Hormone Deficiency.Citation16,Citation24,Citation29
Table 3 The Predicted Difference Between Observed IGF-I SDS and Average IGF-I SDS and Predicted Ratio Between IGF-I and Average IGF-I Concentrations by timeCitation25
Table 4 Recommended Dosing for Patients Prescribed Doses of 0.24 mg/kg/Week Based Upon Available Cartridge sizeCitation39
Figure 3 Skytrofa electronic delivery device and its features.Citation39

