Figures & data
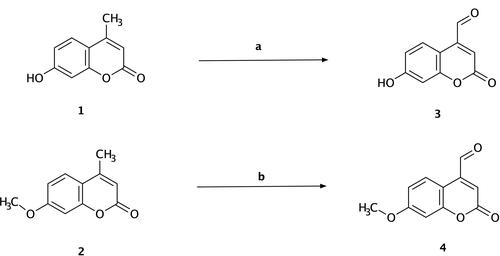
Scheme 1 Synthesis of 7-hydroxy-4-formyl coumarin (3) and 7-methoxy-4-formyl coumarin (4). Reagents and conditions: (a) 7-hydroxy-4-methyl coumarin (1), hot xylene, selenium dioxide, reflux for 12 h, 135–140 °C, filtration, yield = 22%; (b) 7-methoxy-4-methyl coumarin (2), hot xylene, selenium dioxide, reflux for 12 h, 135–140 °C, filtration, yield = 31%.
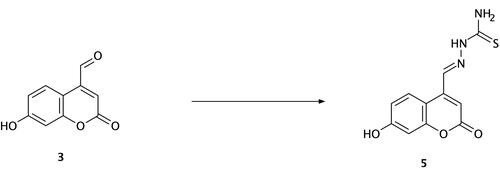
Scheme 2 Synthesis of compound (5) (thiosemicarbazide Schiff base derivative). Reagents and conditions: 7-hydroxy-4-formyl coumarin (3), thiosemicarbazide, ethanol, reflux for 7 h, 75–80 °C, filtration and recrystallization, yield = 67%.
Scheme 3 Synthesis of compounds (6), (7), and (8). Reagents and conditions: (a) 7-methoxy-4-formyl coumarin (4), 4-aminobenzoic acid, ethanol, reflux for 7 h, 75–80 °C, filtration and recrystallization, yield = 87%; (b) 7-methoxy-4-formyl coumarin (4), 5-amino-1,3,4-thiadiazole-2-thiol, ethanol, reflux for 7 h, 75–80 °C, filtration and recrystallization, yield = 56%; (c) 7-methoxy-4-formyl coumarin (4), 4-aminoacetophenone, ethanol, reflux for 7 h, 75–80 °C, filtration and recrystallization, yield = 71%.
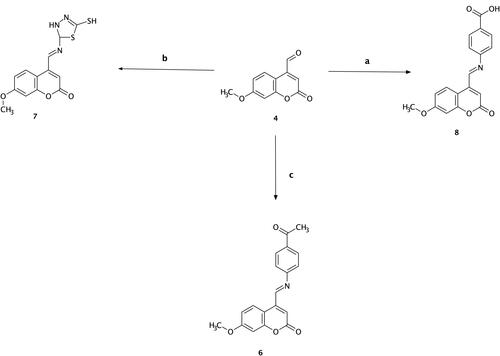
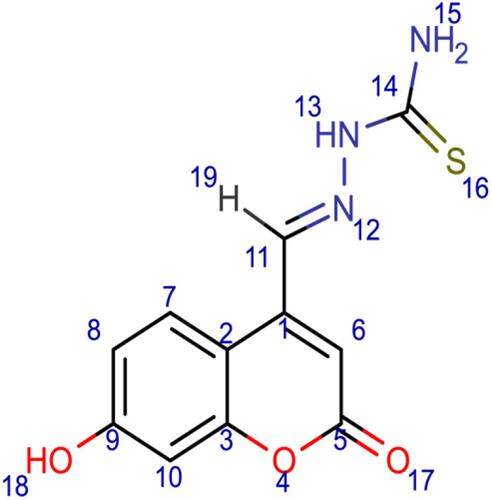
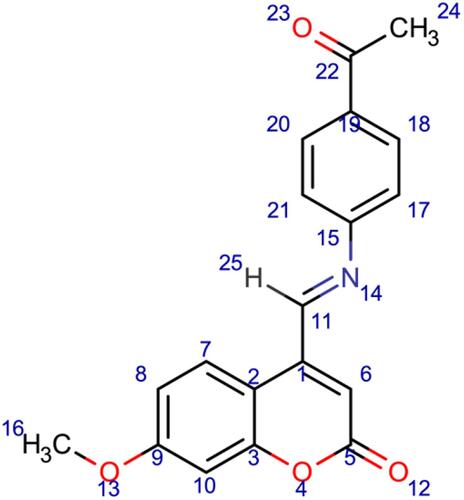
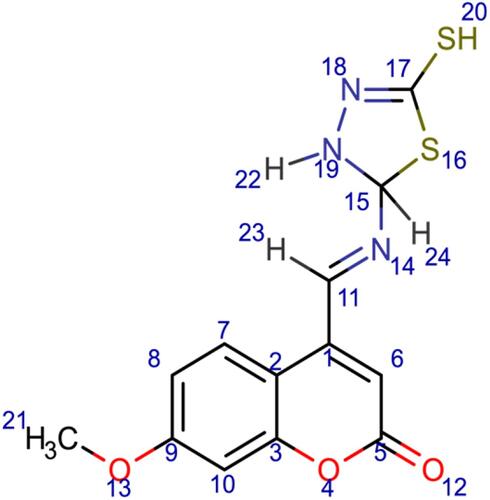
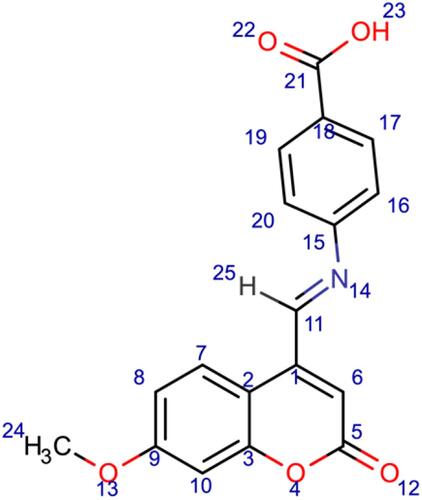
Figure 1 Chemical structures of the synthesized coumarin Schiff base derivatives. Compound 3, 7-hydroxy-4-formyl coumain; compound 4, 7-methoxy-4-formyl coumarin; compound 5, thiosemicarbazide derivative of 7-hydroxy-4-formyl coumarin; compound 6, p-aminoacetophenone derivative of 7-methoxy-4-formyl coumarin; compound 7, 5-amino-1,3,4-thiadiazole-2-thiol derivative of 7-methoxy-4-formyl coumarin; compound 8, p-aminobenzoic acid derivative of 7-methoxy-4-formyl coumarin.
Table 1 Calculated Binding Free Energy, Ki, Run Number of the Highest Δgbinding, LogP, H-Bond Donor, H-Bond Acceptor, and Molar Mass of the Designed Molecules and Ibuprofen
Figure 2 Binding interactions between ibuprofen and the COX-2 binding site. (A) Conformation of ibuprofen in the receptor-binding pocket. (B) The intermolecular binding interactions between ibuprofen and COX-2 presented by Ligplot plus program. The hydrophobic interactions between the ligand and COX-2, red lines; H-bonds, green lines; and protein residues involved in hydrophobic contacts, red spikes.
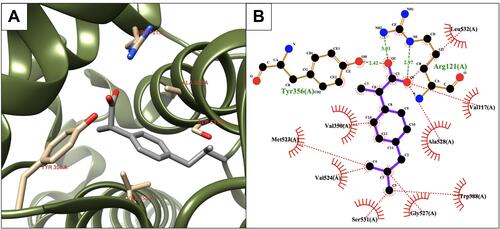
Figure 3 Binding interactions between compound 5 and the COX-2 binding site. (A) Conformation of the ligand in the receptor active site. (B) Intermolecular binding interaction displayed by Ligplot plus program. Hydrophobic bonds between compound 5 and COX-2, red dashed lines; H-bonds, green dashed lines; and protein residues involved in hydrophobic interactions, red arcs.
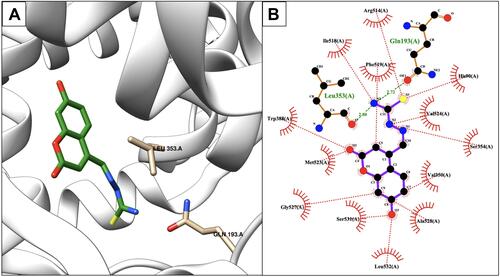
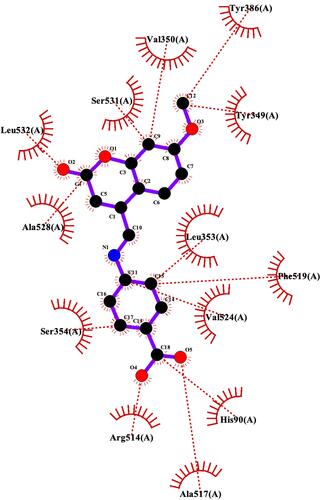
Figure 4 Binding interactions between compound 7 and COX-2 isoform. (A) Conformation of compound 7 in the receptor active pocket. (B) Demonstration of the binding interactions between compound 7 and COX-2. H-bonds, green dashed lines; receptor residues involved in hydrophobic contacts, red arcs; and ligand-protein hydrophobic interactions, red dotted lines.
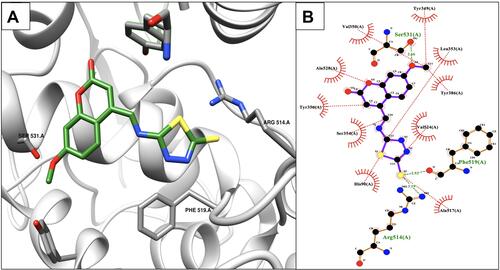
Figure 6 Protein denaturation assay of the synthesized ligands (Schiff base derivatives) and ibuprofen based on the compound concentration. Values represent means of triplicate readings. Ibu, Ibuprofen; 5, compound 5; 6, compound 6; 7, compound 7; 8, compound 8.
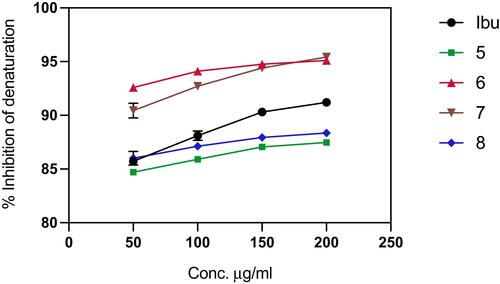
Table 2 Pearson’s Correlation, Coefficient of Determination, and the Level of Significance for the Schiff Base Derivative Ligands and Ibuprofen