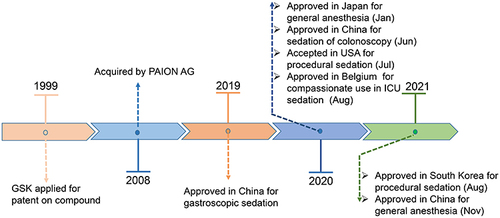
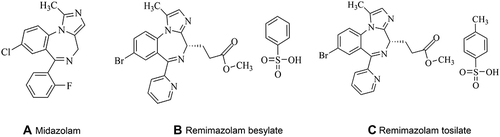
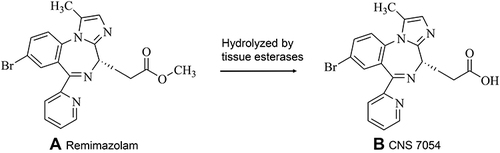
Figures & data
Table 1 Pharmacokinetic Parameters of Remimazolam and Midazolam
Figure 4 The context-sensitive half-times (the time required for the plasma level of the drug to decrease 50% after the infusion is stopped) for the sedatives remimazolam, midazolam, propofol, etomidate, and dexmedetomidine.
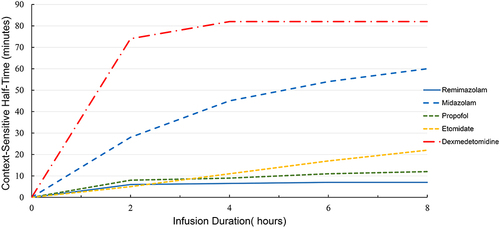
Table 2 Main Clinical Trials of Remimazolam for Sedation Under Gastroscopy
Table 3 Main Clinical Trials of Remimazolam for Sedation Undergoing Colonoscopy
Table 4 Main Clinical Trials of Remimazolam for Sedation Under Bronchoscopy
Table 5 Main Clinical Trials of Remimazolam for Sedation in General Anesthesia
Table 6 Main Articles About Other Clinical Application of Remimazolam