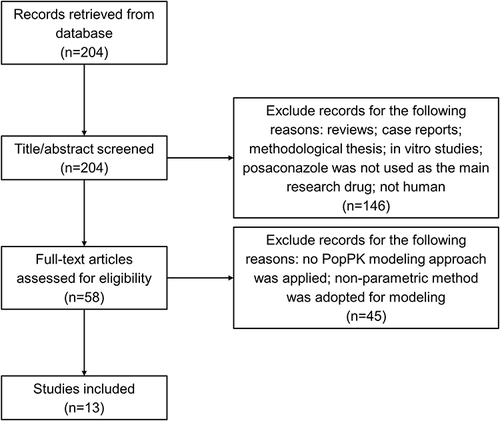
Figures & data
Table 1 Population Characteristics of the Studies Included in the Review
Table 2 Model Characteristics of the Studies Included in the Review
Table 3 Results from Published Population Pharmacokinetic Models of Posaconazole
Figure 2 Simulated steady-state concentration-time profiles at different covariate levels for the reported PopPK models. (A) oral suspension; (B) tablets and intravenous formulations. The dashed line corresponds to a plasma steady state concentration of 0.7 mg/L, and the dash-dotted line corresponds to a plasma steady state concentration of 1.0 mg/L.
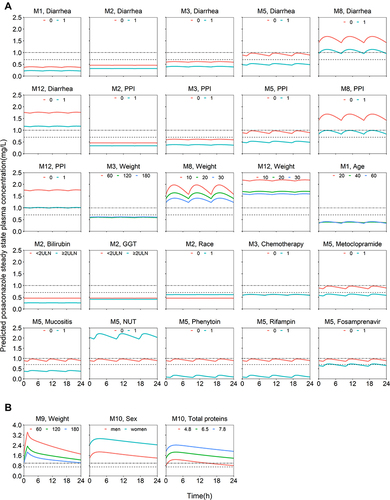
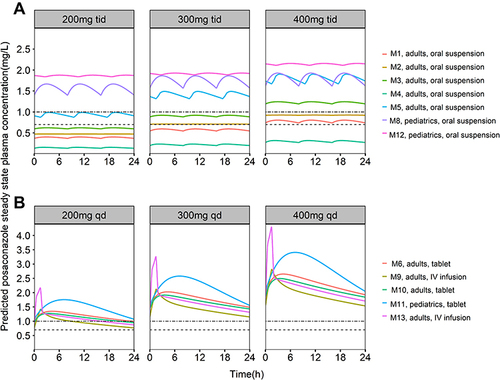
Figure 3 Simulated steady-state concentration-time profiles at different dosing regimens for the reported PopPK models. (A) adults or pediatrics receive posaconazole oral suspension 200, 300, and 400 mg thrice daily; (B) adults or pediatrics receive tablets or intravenous formulations of 200, 300, and 400 mg twice daily on the first day and once daily for maintenance. The dashed line corresponds to a plasma steady state concentration of 0.7 mg/L, and the dash-dotted line corresponds to a plasma steady state concentration of 1.0 mg/L.