Figures & data
Table 1 Drug/ Polymer Composition and Concentration in the Different Formulations of GZ Mucoadhesive Films
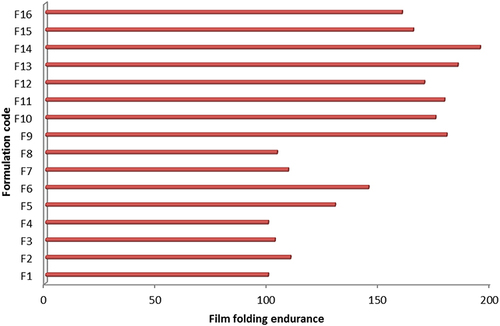
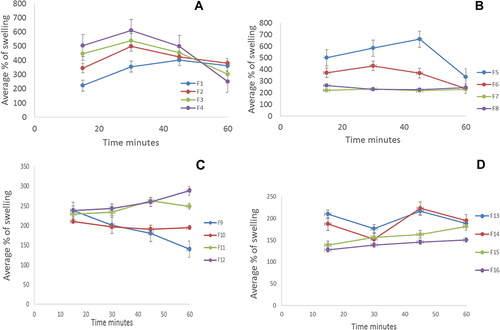
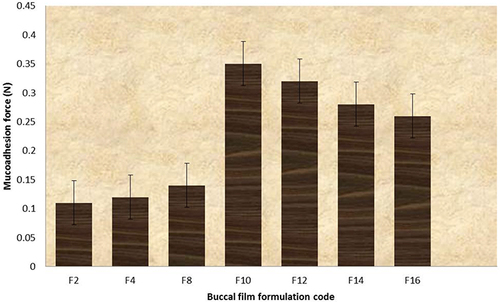
Table 2 Different Physicochemical Parameters of the GZ Bucco-Adhesive Films
Figure 8 Release profile of GZ from selected buccal films (F2, F4, F8, F10, F12, F14 and F16) in 0.1N HCl buffer for 2 hours.
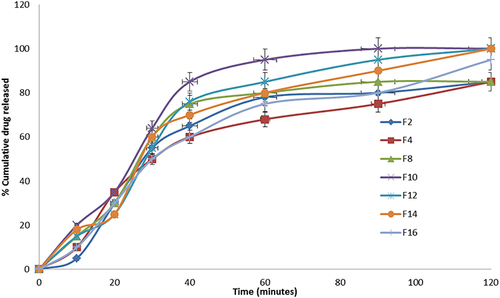
Table 3 Stability of Selected GZ Films (F10, F12, F14) in Term of Folding Endurance, Moisture Content, Drug Content and Muco-Adhesion Strength at 4°C, 25°C, and 40°C After 90 Days
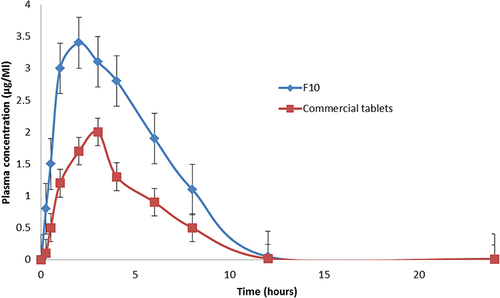
Table 4 Mean Pharmacokinetic Parameters of GZ After Buccal Use of F10, and Oral Administration of Commercial Tablets to Rabbits