Figures & data
Figure 1 Chart showing the circadian rhythm of cortisol production as well as levels of IL-6 in RA patients.
Abbreviations: IL-6, interleukin 6; MR, modified-release; RA, rheumatoid arthritis.
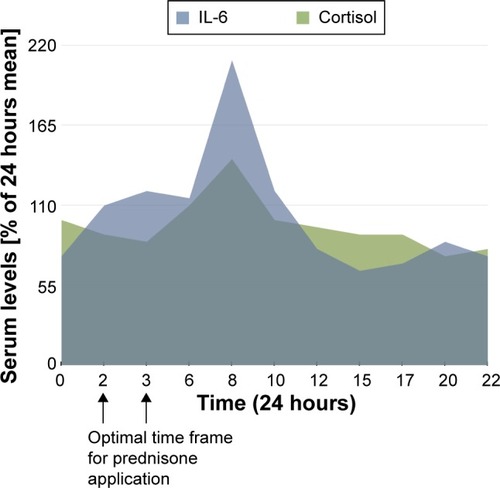
Figure 2 Simplified diagram showing ingestion, liberation, and way of action of modified-release prednisone.
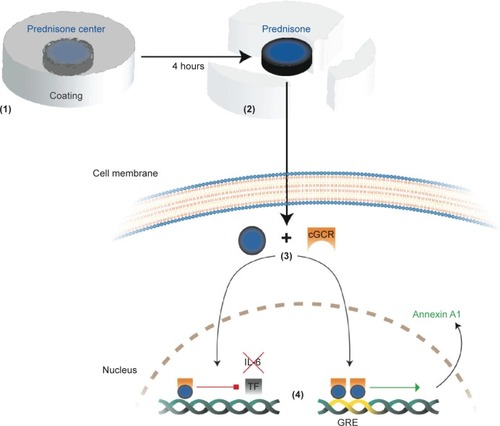
Table 1 Details of the most important clinical trials or evaluations of trial data on MR prednisone so far
Table 2 AEs reported in the clinical trials comparing MR prednisone with IR prednisone and placebo, respectivelyCitation36,Citation38,Citation39
