Figures & data
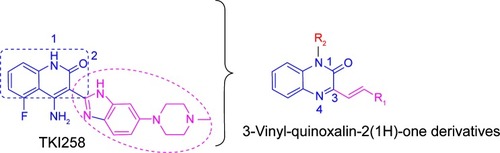
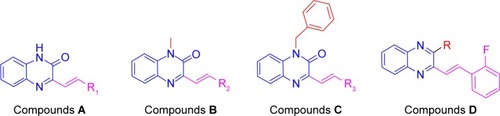
Figure 3 The synthetic pathway for quinoxaline derivatives A1–A14, B1–B5, C1–C5, and D1–D3.
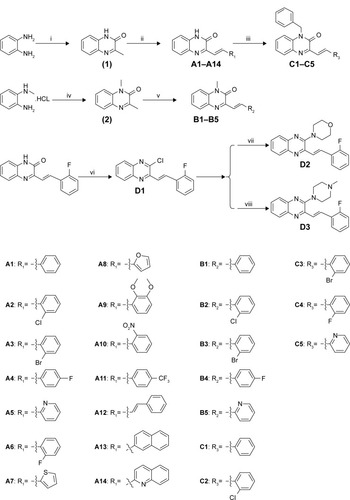
Table 1 Specificity and potency of compounds kinase inhibitor
Figure 4 Kinase inhibition profile for these 27 compounds against FGFR1 at 10, 1, and 0.1 µM.
Abbreviations: DMSO, dimethyl sulfoxide; SEM, standard error of the mean.
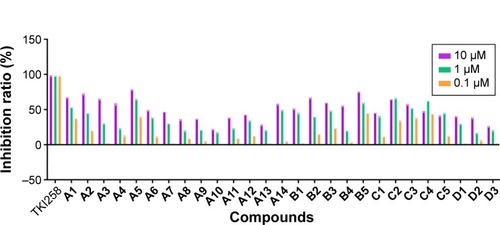
Table 2 Cellular antiproliferative activity
Figure 5 Relative cell viability of HL7702 cells by compounds (TKI258 and A5) treatment at 2 (A) and 10 µM (B) as illustrated above.
Abbreviations: DMSO, dimethyl sulfoxide; SEM, standard error of the mean.
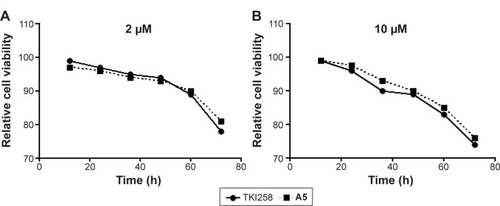
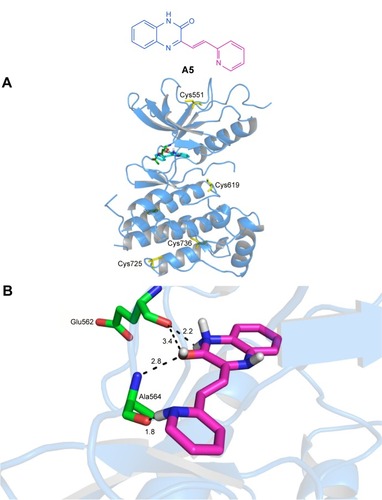
Figure 6 Molecular docking of compound A5 and FGFr1.