Figures & data
Figure 1 Effects of intrastriatal administration of Tat-fusion interfering peptide (Tat-D1Ri) on D1R–GluN1 interactions.
Abbreviations: Tat-D1Rc, Tat-fusion control peptide; Ig, Immunoglobulin; IP, immunoprecipitation; IB, immunoblot.
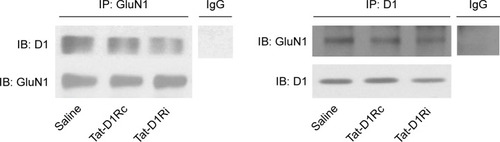
Figure 2 Effects of intrastriatal administration of Tat-D1Ri on established dyskinetic behaviors in 6-OHDA-lesioned rats.
Abbreviations: 6-OHDA, 6-hydroxydopamine; l-dopa, L-3,4-dihydroxyphenylalanine; LID, l-dopa-induced dyskinesia; SEM, standard error of mean; Tat-D1Ri, Tat-fusion interfering peptide; Tat-D1Rc, Tat-fusion control peptide; ALO AIM, axial, limb, and orolingual movements.
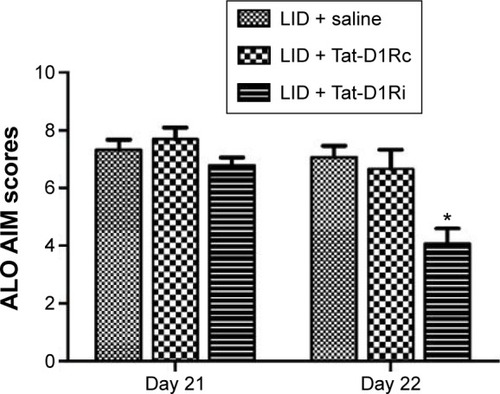
Figure 3 Effects of intrastriatal administration of Tat-D1Ri on DARPP-32 phosphorylation level after levodopa treatment in 6-OHDA-lesioned rats.
Abbreviations: 6-OHDA, 6-hydroxydopamine; DARPP-32, dopamine- and cAMP-regulated phosphoprotein of 32 kDa; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; l-dopa, L-3,4-dihydroxyphenylalanine; LID, l-dopa-induced dyskinesia; P-DARPP-32, phosphorylated DARPP-32; SEM, standard error of mean; Tat-D1Ri, Tat-fusion interfering peptide; Tat-D1Rc, Tat-fusion control peptide; PD, Parkinson’s disease.
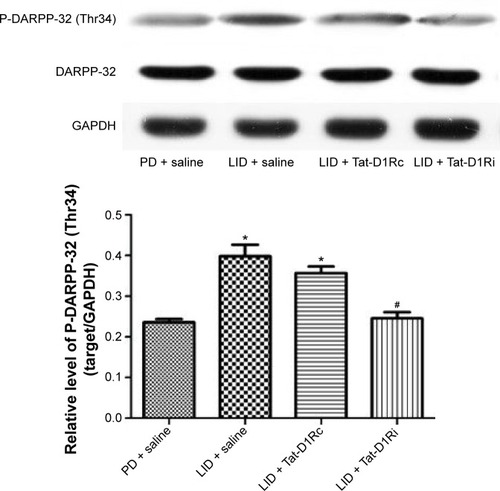
Figure 4 Effects of intrastriatal administration of Tat-D1Ri on membrane NMDA subunit expression.
Abbreviations: 6-OHDA, 6-hydroxydopamine; l-dopa, L-3,4-dihydroxyphenylalanine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LID, l-dopa-induced dyskinesia; SEM, standard error of mean; Tat-D1Ri, Tat-fusion interfering peptide; Tat-D1Rc, Tat-fusion control peptide; NMDA, N-methyl-d-aspartate; PD, Parkinson’s disease.
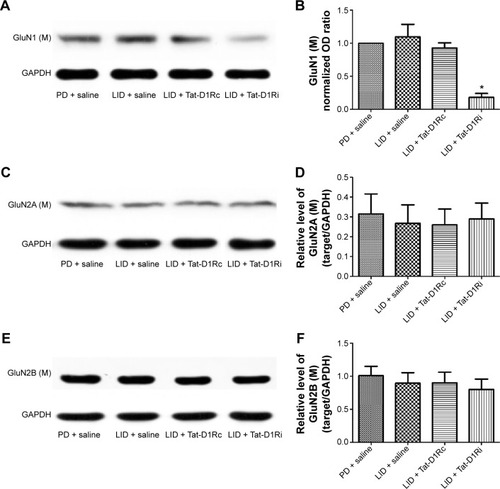
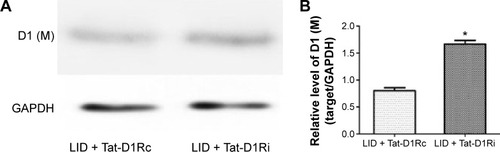
Figure 5 Effects of intrastriatal administration of Tat-D1Ri on membrane D1R subunit expression.
Abbreviations: 6-OHDA, 6-hydroxydopamine; l-dopa, L-3,4-dihydroxyphenylalanine; LID, l-dopa-induced dyskinesia; SEM, standard error of mean; Tat-D1Ri, Tat-fusion interfering peptide; Tat-D1Rc, Tat-fusion control peptide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.