Figures & data
Figure 1 The survey of the structural characteristics of staurosporine, BPIC, and MIAM, as well as the docking scores results in BHIMHA as a novel inhibitor of PKCα (PDB: 3IW4) capable of inhibiting metastasis of tumor toward lung, slowing tumor growth, and blocking inflammatory response.
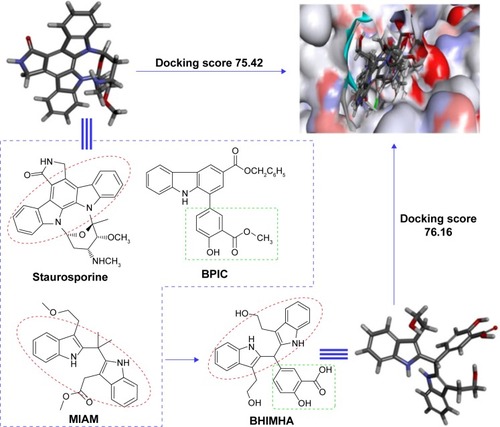
Figure 2 Synthesis route to BHIMHA.
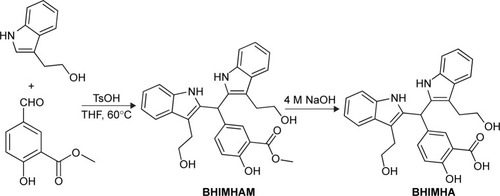
Figure 3 Docking investigation of BHIMHA and staurosporine toward PKCα (PDB: 3IW4).
Abbreviations: PKC, protein kinase C; BHIMHA, 5-(bis(3-(2-hydroxyethyl)-1H-indol-2-yl)-methyl)-2-hydroxybenzoic acid; staurosporine, (5S,6R,7R,9R)-6-methoxy-5-methyl-7-methylamino-6,7,8,9,15,16-hexahydro-5H,14H-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b, h]cyclone-na[jkl]cyclopenta[e]asindacen-14-one; PDB, Protein Data Bank.
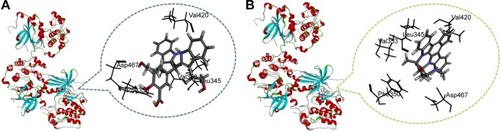
Figure 4 Effect of BHIMHA on migration and invasion of A549 cells (purple particles).
Abbreviations: SD, standard deviation; BHIMHA, 5-(bis(3-(2-hydroxyethyl)-1H-indol-2-yl)-methyl)-2-hydroxybenzoic acid.
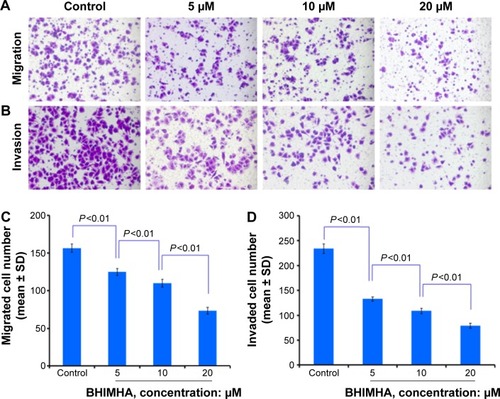
Figure 5 In vivo BHIMHA dose dependently inhibits the metastasis of tumor toward lung.
Abbreviations: NS, normal saline; LLC, Lewis lung carcinoma; PKCα, protein kinase Cα; BHIMHA, 5-(bis-(3-(2-hydroxyethyl)-1H-indol-2-yl)methyl)-2-hydroxybenzoic acid; staurosporine, (5S,6R,7R,9R)-6-methoxy-5-methyl-7-methylamino-6,7,8,9,15,16-hexahydro-5H,14H-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b, h]cyclonona[jkl] cyclopenta[e]asindacen-14-one.
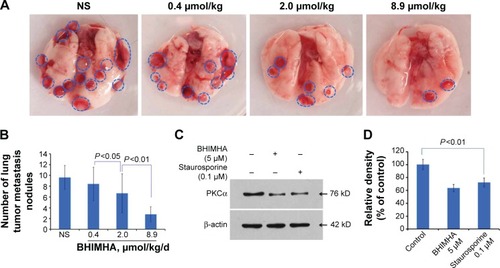
Figure 6 In vivo oral BHIMHA dose dependently inhibits the growth of the primary tumor of LLC planted C57BL/6 mice (n=10).
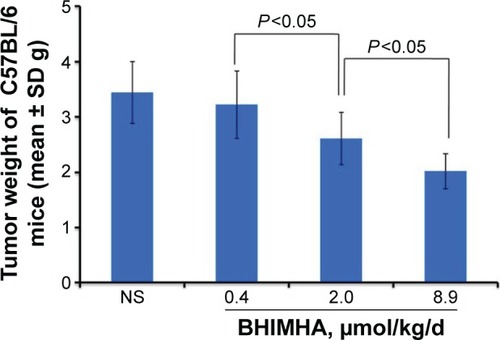
Figure 7 In vivo activities of BHIMHA, Dox and NS in slowing tumor growth of S180 mice, n=12.
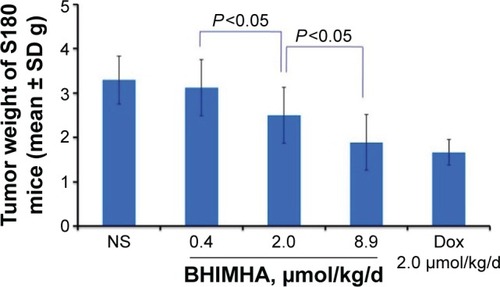
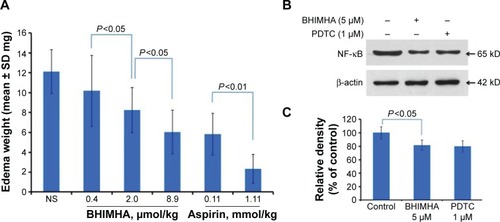
Figure 8 (A) In vivo activity of BHIMHA inhibiting xylene-induced ear edema of ICR mice, n=10; (B, C) effect of BHIMHA on the expression of NF-κB from A549 cells, n=3.