Figures & data
Table 1 Phase III clinical trials of FDA-approved drugs for metastatic castration-resistant prostate cancer
Figure 1 Three mechanisms of action of galeterone.
Abbreviation: Enz, enzalutamide.
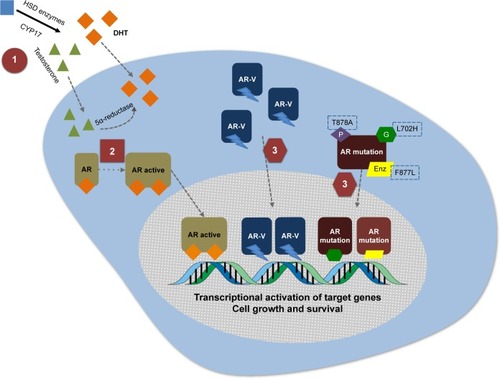
Table 2 Main characteristics and outcomes of the ARMOR1 and ARMOR2 clinical trials
Table 3 Main adverse events in the ARMOR1 and ARMOR2 clinical trials
Table 4 ARMOR2: galeterone efficacy summary
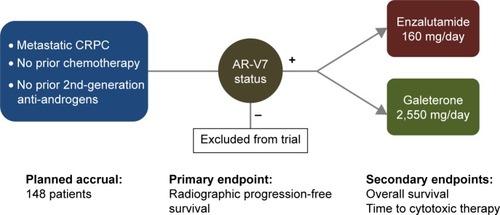
Figure 2 ARMOR3-SV: Phase III randomized trial design and study endpoints.
Abbreviations: CRPC, castration-resistant prostate cancer; AR-V7, androgen receptor splice variant 7.