Figures & data
Table 1 Demographic data and baseline characteristics
Table 2 Summary of pharmacokinetic parameters of 5-ASA and Ac-5-ASA in children and adolescents
Figure 2 Mean (SD) plasma concentration–time profiles for (A) 5-ASA and (B) Ac-5-ASA in children and adolescents by treatment group.
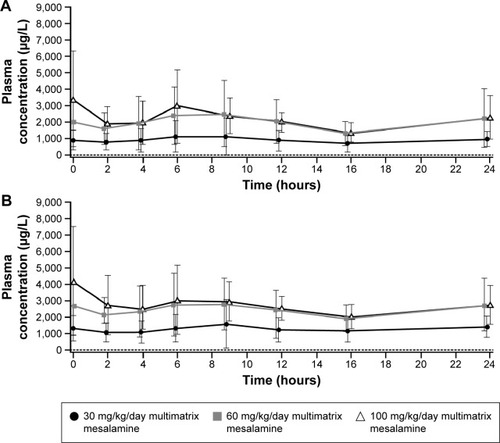
Figure 3 Box and whisker plots of simulated 5-ASA and Ac-5-ASA steady-state AUC in both child/adolescent and adult populations for low-dose (A [5-ASA] and C [Ac-5-ASA]) and high-dose exposures (B [5-ASA] and D [Ac-5-ASA]).
![Figure 3 Box and whisker plots of simulated 5-ASA and Ac-5-ASA steady-state AUC in both child/adolescent and adult populations for low-dose (A [5-ASA] and C [Ac-5-ASA]) and high-dose exposures (B [5-ASA] and D [Ac-5-ASA]).](/cms/asset/f184af5a-36d1-4204-8324-3bd0a069c9fb/dddt_a_12182234_f0003_b.jpg)
Table 3 Treatment-emergent adverse events
Figure S1 Schematic of final population pharmacokinetic structural model.
Abbreviations: ALAG1, absorption lag time from depot 1; 5-ASA, 5-aminosalicylic acid; Ac-5-ASA, acetyl-5-aminosalicylic acid; Ka1, absorption rate constant from depot 1; F1, fraction of dose absorbed from depot 1; Ka3, absorption rate constant from depot 3; ALAG3, absorption lag time from depot 3 in addition to the lag time from depot 1; CLM, metabolic clearance of 5-ASA; CLNRM, non-renal clearance of Ac-5-ASA; CLR, renal clearance of 5-ASA; CLRM, renal clearance of Ac-5-ASA.
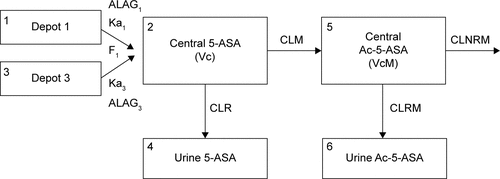
Figure S2 Goodness-of-fit and diagnostic plots for the final model in children and adolescents: plasma 5-ASA.
Abbreviation: 5-ASA, 5-aminosalicylic acid.
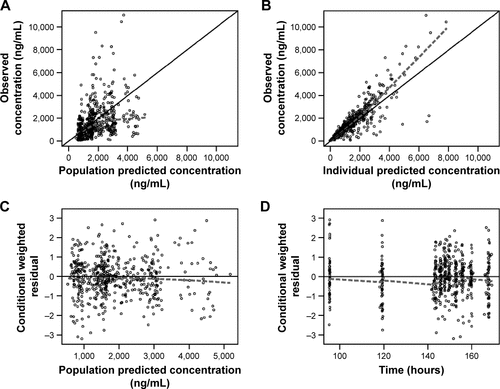
Figure S3 Goodness-of-fit and diagnostic plots for the final model in children and adolescents: plasma Ac-5-ASA.
Abbreviation: Ac-5-ASA, acetyl-5-aminosalicylic acid.
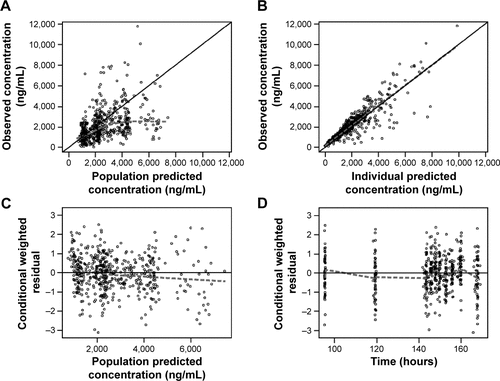
Table S1 Assay performance of 5-ASA and Ac-5-ASA bioanalytical quality control samples in human plasma and urine
Table S2 Parameter estimates of final 5-ASA/Ac-5-ASA population pharmacokinetic modelTable Footnotea
Table S3 Simulated steady-state 5-ASA exposures by weight and dose group
Table S4 Simulated steady-state Ac-5-ASA pharmacokinetic parameters by dose and age group