Figures & data
Table 1 Tested TCM extracts (number)
Figure 1 Misfolded aggregation by ΔK280 tauRD in HEK293T cells.
Abbreviations: CMV, cytomegalovirus; HEK, human embryonic kidney; ΔK280, deletion of the lysine residue at codon 280; tauRD, tau repeat domain.
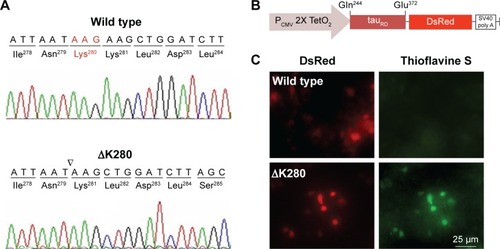
Figure 2 Tet-On ΔK280 tauRD-DsRed 293 cell model for AD.
Abbreviations: AD, Alzheimer’s disease; Dox, doxycycline; PCR, polymerase chain reaction; HPRT1, hypoxanthine phosphoribosyl transferase 1; H33342, Hoechst 33342; tauRD, tau repeat domain.
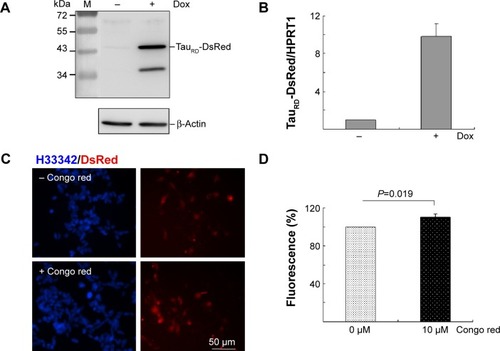
Figure 3 High-content screening of TCM extracts.
Abbreviations: Dox, doxycycline; HCA, high-content analysis; ROS, reactive oxygen species; SD, standard deviation; TCM, traditional Chinese medicine.
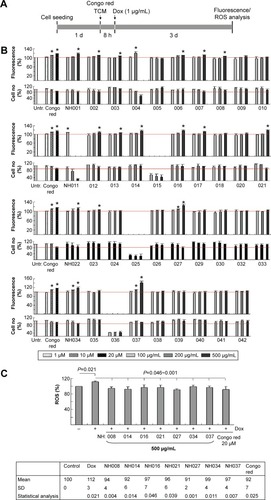
Figure 4 Cytotoxicity of the selected TCM extracts.
Abbreviations: HEK, human embryonic kidney; IC50, half maximal inhibitory concentration; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; TCM, traditional Chinese medicine.
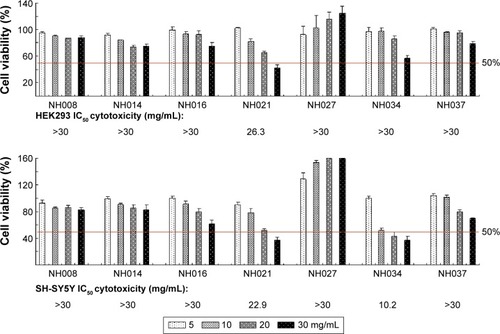
Figure 5 Neuroprotective effects of the selected TCM extracts in SH-SY5Y ΔK280 tauRD-DsRed cells.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Dox, doxycycline; SD, standard deviation; tauRD, tau repeat domain; TCM, traditional Chinese medicine; TUBB3, tubulin, beta 3 class III.
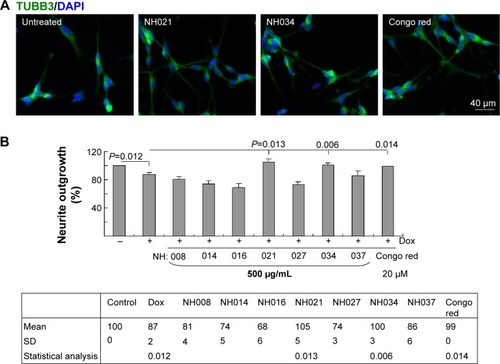
Table 2 Genes identified by the unfolded protein response plus PCR array
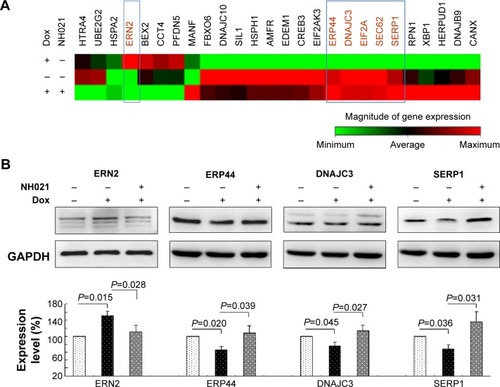
Figure 6 Alterations of UPR-related gene expression in Tet-On ΔK280 tauRD-DsRed SH-SY5Y cells.
Abbreviations: Dox, doxycycline; DNAJC3, DnaJ (Hsp40) homolog, subfamily C, member 3; ERN2, endoplasmic reticulum to nucleus signaling 2; ERP44, endoplasmic reticulum protein 44; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SERP1, stress-associated endoplasmic reticulum protein 1; tauRD, tau repeat domain; UPR, unfolded protein response.