Figures & data
Table 1 MetaGeM observational studies and patients included in the analysis
Table 2 Demographic characteristics of patients
Table 3 Specific medications taken at inclusion in the study
Table 4 AEs according to system organ class (MedDRA)
Table 5 Occurrence of AEs during the study
Figure 1 Evolution of AEs by gender.
Abbreviation: AEs, adverse events.
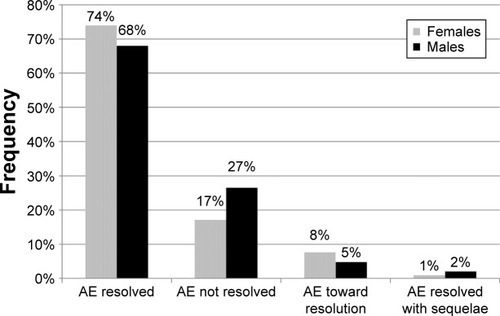
Table 6 Serious adverse events (SAEs) occurred during study
