Figures & data
Figure 1 The canonical model of insulin secretion in pancreatic beta-cells. When the plasma glucose concentration rises to 5mM or higher, glucose transporter 2 (GLUT2), an insulin-insensitive glucose transporter, allows glucose to enter islet beta-cells from the circulation. The product of glycolysis is pyruvate which increases tricarboxylic acid cycle turnover and oxidative phosphorylation. This results in a rise in the cell’s ATP/ADP ratio which leads to the closure of ATP-sensitive potassium channels, causing membrane depolarisation (due to potassium becoming sequestered in the cell) and subsequent calcium influx. The resulting calcium influx then triggers insulin secretion by causing insulin vesicles to fuse with the plasma membrane. ROS produced during oxidative phosphorylation have been shown to amplify insulin secretion, although it is not understood how they do so mechanistically. This figure and the information in its legend are adapted from these studies.Citation41,Citation48,Citation53,Citation55
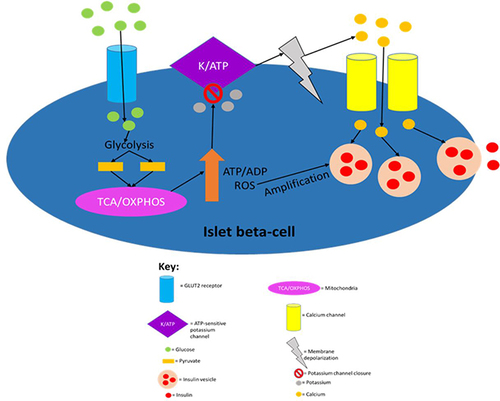
Figure 2 A summary of how proglucagon is alternatively processed in different tissues to produce the desired products. Proglucagon undergoes alternative processing by prohormone convertase enzymes in islet alpha-cells, L-cells of the gut and neuronal cells. Glucagon and GLP-1 are both pivotal hormones needed to maintain metabolic homeostasis. Only the biologically active products are shown and just GLP-1 and glucagon activity are discussed in this review. This figure and the information in its legend are adapted from these studies.Citation26,Citation107,Citation108,Citation112
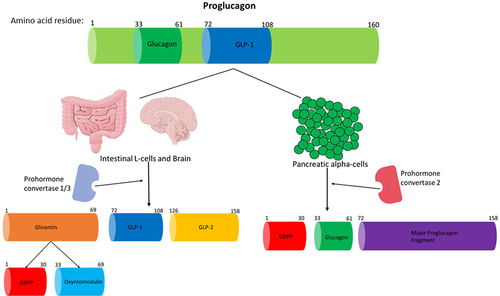
Figure 3 Regulation of glucagon secretion in pancreatic alpha-cells. Sodium-coupled GLUT2 receptor allows glucose to enter islet alpha-cells in an inuslin independent manner. When glucose is absent or at low levels in circulation, glycolysis and mitochondrial ATP production is greatly decreased leading to a decreased intracellular ATP/ADP ratio. This then allows for ATP-dependent K+ channels to be open, as ATP molecules block this channel. Opening of the ATP-dependent K+ channels then allows for intracellular potassium efflux which causes membrane depolarisation to an electrical range that permits opening of Ca2+ channels, which in turn, allows for extracellular calcium influx. The raised intracellular calcium levels then trigger glucagon secretion by causing glucagon vesicles to fuse with the plasma membrane. This figure and the information in its legend are adapted from these studies.Citation34,Citation110,Citation111
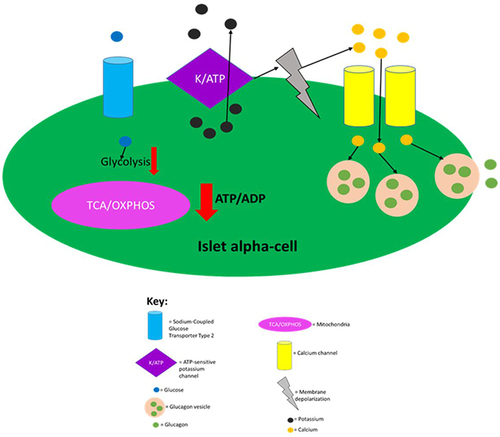
Figure 4 A summary of the effects that insulin and glucagon have on various organs after they have been released in response to the fed or fasting state, respectively. Both hormones have direct and indirect (highlighted red) effects on all of these organs. This figure and the information in its legend are adapted from these studies.Citation4,Citation8,Citation34,Citation40,Citation77,Citation78,Citation80,Citation104,Citation116–119
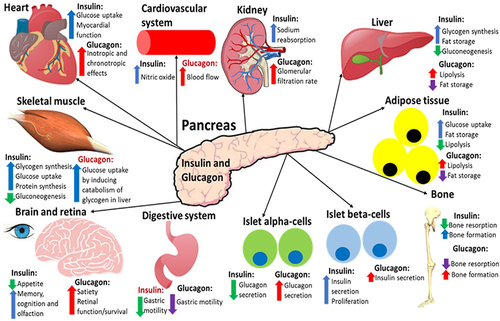
Figure 5 A summary of the processes in L-cells that are known to lead to/may lead to GLP-1 secretion into the circulation. Sodium entry into the L-cell promotes calcium influx by inducing depolarisation and glucose and fatty acids also induce calcium influx by raising ATP levels as a result of their catabolism. The now elevated calcium levels promote exocytosis of GLP-1-containing vesicles, resulting in GLP-1 being released into circulation. Proteins also promote GLP-1 release, but it is not currently mechanistically understood how. This figure is reproduced from (Reed J, Bain S, Kanamarlapudi V. Recent advances in understanding the role of glucagon-like peptide 1. F1000Research. 2020; 9(239):1–14; Creative Commons)Citation32 and the information in its legend are adapted from these studies.Citation194–199
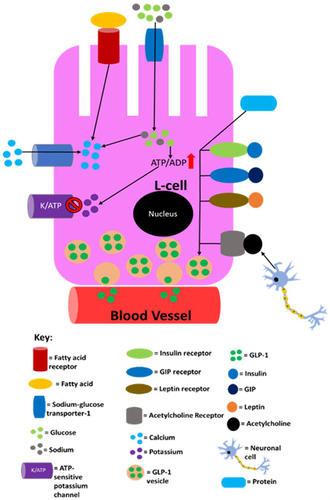
Figure 6 An overview of the effects/possible effects that GLP-1 has on various organs after it has been secreted into the circulation from L-cells of the gut in response to nutrient ingestion. Organs highlighted in blue are not known to express GLP-1R but GLP-1 has been demonstrated to mediate insulin-like effects on these tissues during experimental settings- it is, however, correct to state that GLP-1 does have indirect effects on these organs in humans due to its ability to promote the incretin effect. GLP-1R mRNA has been detected in immune cells and GLP-1 has been shown to regulate the immune system (highlighted in red) activity during experimental settings. GLP-1 has also been shown to influence bone (highlighted green) metabolism in rodents but the effects of GLP-1 on human bone are currently elusive. This figure and the information in its legend are adapted from these studies.Citation11,Citation26,Citation107,Citation108,Citation112,Citation120,Citation191
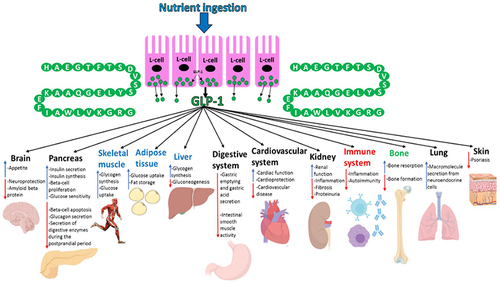
Figure 7 An overview of the processes in islet beta-cells that mediate incretin-induced insulin secretion. Glucose entry into islet beta-cells from the blood circulation via GLUT2 undergoes catabolism to raise ATP levels, which then induce the closure of ATP-sensitive potassium channels, causing membrane depolarisation and subsequent calcium influx. The resulting calcium influx then triggers insulin secretion. The binding of GLP-1 and GIP, which are secreted from the intestine into the circulation in response to nutrient ingestion, to GLP-1R and GIPR, respectively, enhances glucose-stimulated insulin secretion by initiating cyclic adenosine monophosphate (cAMP) production via activation of adenylyl cyclase by Gα, which, in turn, activates protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac). PKA and Epac further promote potassium channel closure which indirectly assists with extracellular calcium influx. Also, Epac directly induces calcium release from the endoplasmic reticulum, whereas PKA is thought to increase the permeability of calcium channels to enable a more rapid influx of calcium. The now elevated intracellular calcium levels further enhance the exocytosis of insulin vesicles. GLP-1R activation also induces transcription of the preproinsulin, glucokinase and GLUT2 genes allowing for further insulin production, glucose catabolism and glucose uptake, respectively, via activation of the PDX-1 transcription factor and its translocation to the nucleus. PDX-1 activation also induces transcription of genes involved in proliferation, neogenesis and apoptotic resistance. Additionally, GLP-1R activation promotes intracellular lipid catabolism which is thought to provide the mitochondria with more metabolic fuel to further raise the ATP/ADP ratio- ATP is needed for both phases of insulin secretion. This figure and the information in its legend are adapted from these studies.Citation11,Citation55,Citation107,Citation112,Citation120,Citation191
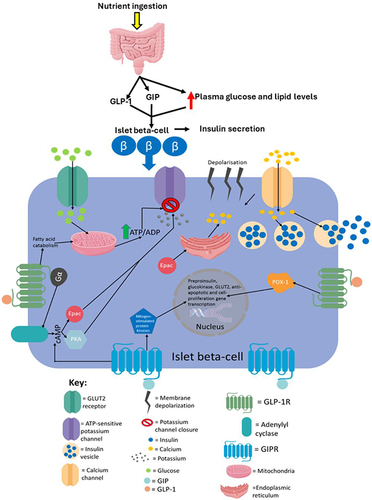
Figure 8 A summary of the direct effects that GIP has on various organs expressing GIPR upon its release during the postprandial period. This figure and the information in its legend are adapted from these studies.Citation119,Citation120,Citation308–311,Citation314,Citation315,Citation317,Citation321,Citation325,Citation330–334
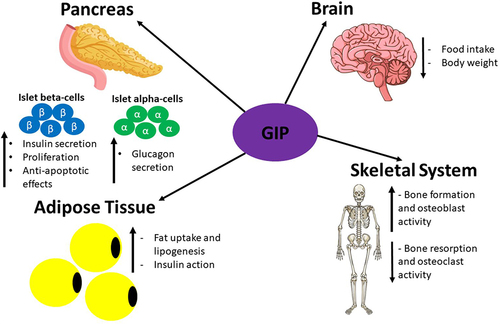
Table 1 Current GLP-1R agonist used in T2D treatment and their reported effects on cardiovascular and kidney pathology
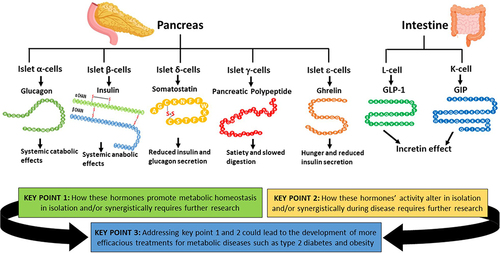
Figure 9 The hormones discussed in this review and the key points raised. The hormones involved in the regulation of metabolic homeostasis produced by the pancreas and the GIT discussed in this review are shown with their main/established effects. The key points of this review are displayed here, to highlight the potential importance of further understanding these hormones’ actions in isolation and synergistically during healthy and disease states could generate more efficacious and highly desirable treatments for metabolic disorders, such as T2D and obesity. This figure and the information in its legend are adapted from these studies.Citation26,Citation32,Citation42,Citation48,Citation107,Citation120,Citation143,Citation148,Citation164,Citation165