Figures & data
Figure 1 Patterns of trans-synaptic degeneration in the afferent visual pathway in multiple sclerosis. The visual pathway is a functionally eloquent sensory pathway made up of 3 neurons, travelling from the retina to the primary visual cortex of the occipital lobe. The axons of the 2nd order neurons (travelling from the retinal ganglion cell layer through the optic nerve and optic tracts to the thalami) are highly organized, with the axons from the nasal half of the retina crossing over to the contralateral cerebral hemisphere within the optic chiasm, while the axons from the temporal half of the retina remain uncrossed and continue their path within the ipsilateral cerebral hemisphere. The crossed and uncrossed nature of the pathway means that each thalamus and visual cortex receives inputs from the right and left eye in a homonymous pattern. In optic neuritis, injury to the axons of the 2nd order neurons (prior to the optic chiasm) can result in anterograde degeneration of affected axons, a process which may then proceed trans-synaptically, resulting in anterograde trans-synaptic degeneration of the 3rd order neurons travelling from both thalami to both primary visual cortices. Neuroaxonal degeneration after optic neuritis may also proceed in the retrograde direction, resulting in loss of cell bodies in the ipsilateral retinal ganglion cell layer (GCL), and potentially trans-synaptically to the 1st order neurons contained in the ipsilateral retinal inner nuclear layer (INL). On the other hand, if a demyelinating lesion causes axonal injury to the 3rd order neurons (eg within the optic radiations), anterograde neuroaxonal degeneration may result in atrophy of the ipsilateral visual cortex, while retrograde degeneration may proceed to the ipsilateral thalamus, and potentially trans-synaptically to the highly organized crossed and uncrossed 2nd order neurons, resulting in retrograde trans-synaptic degeneration with a homonymous pattern of atrophy of the retinal ganglion cell layer (and possibly even trans-synaptically again to the 1st order neurons within the retinal inner nuclear layer). Reproduced from Murphy OC, Calabresi PA, Saidha S. Illustrations of the Afferent Visual Pathway and Concepts Surrounding Trans-Synaptic Neuroaxonal Degeneration in the Visual Pathway in Multiple Sclerosis.Citation5
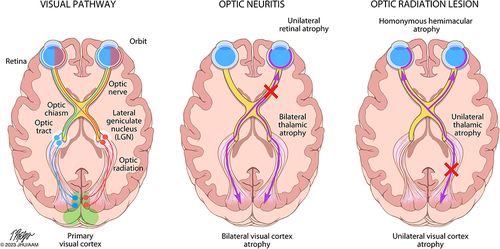
Table 1 Summary of Evidence in Support of Anterograde and Retrograde Trans-Synaptic Degeneration in the Visual System in MS
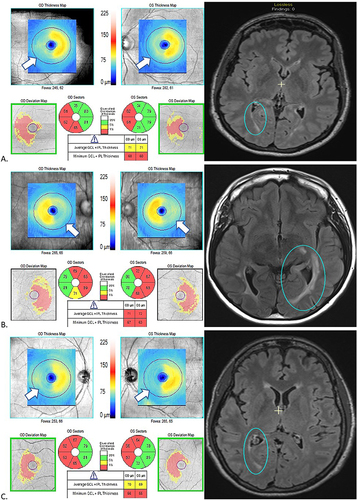
Figure 2 MRI and OCT findings in three patients (A, B, C) with multiple sclerosis and homonymous, hemi-macular ganglion cell + inner plexiform layer (GCIPL) atrophy in conjunction with posterior visual pathway lesions, a pattern which is thought to be indicative of retrograde trans-synaptic degeneration. In each case (A, B, C), the FLAIR MRI sequence demonstrates a unilateral lesion in the occipital white matter (blue circles), while the macular OCT shows a homonymous, hemi-macular GCIPL thinning with temporal hemi-atrophy in the ipsilateral eye and nasal hemi-atrophy in the contralateral eye. This is demonstrated by the “blue range” hemi-macular color in the thickness map of each OCT scan. In the quantified sectors which provide average GCIPL thickness in each of these sectors, the red sectors correspond to sectors with significant GCIPL thinning (below 1st percentile of healthy individuals of similar age). This is also depicted in the deviation maps, in which thinned areas are delineated in yellow (1st-5th percentile of healthy individuals of similar age) or red (<1st percentile of healthy individuals of similar age).