Figures & data
Figure 1 PRISMA flow diagram. Of the 122 articles identified, 60 articles were excluded. The majority of articles were excluded as they were commentary or discussions or they were irrelevant to the purpose of this review article. Two articles were excluded as they included data for pediatric patients and one excluded as it was a meta-analysis with repetitive data. Sixty articles were screened for inclusion for this review article.
Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.
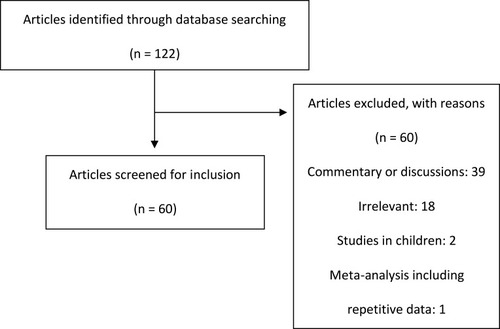
Table 1 Studies of I-R Susceptibilities of Select Gram-Negative Bacteria. MIC50 and MIC90 are the Minimum Inhibitory Concentrations for 50% and 90% of Isolates, Respectively. Imipenem Susceptibility is Based on FDA-Approved MIC Breakpoints as Follows: Enterobacterales, Susceptible (S) ≤1 µg/mL, Intermediate (I) 2 µg/mL, Resistant (R) ≥4 µg/mL; P. aeruginosa, S ≤2 µg/mL, I 4 µg/mL, R ≥8 µg/mL; A. baumannii, S <2 µg/mL, I 4 µg/mL, R >8 µg/mL; Anaerobic Bacteria, S ≤4 µg/mL, I 8 µg/mL, R ≥16 µg/mL
Table 2 I-R Breakpointsa. There are Both FDA-Approved Breakpoints and EUCAST Breakpoints for I-R. For FDA-Approved Breakpoints: Enterobacterales is Susceptible (S) at ≤1 µg/mL, Intermediate (I) at 2 µg/mL, and Resistant (R) at ≥4 µg/mL; P. aeruginosa is S at ≤2 µg/mL, I at 4 µg/mL, and R at ≥8 µg/mL; Anaerobic Bacteria is S ≤4 µg/mL, I at 8 µg/mL, and R at ≥16 µg/mL. For EUCAST Breakpoints, Enterobacterales, P. aerguinosa, Anaerobic Bacteria, and A. baumannii are All S at ≤2 µg/mL and R at >2 µg/mL. Relebactam Concentrations are Fixed at 4 µg/mL
Table 3 Characteristics of I-R. Pharmacokinetics of I-R are Similar to That of Imipenem-Cilastatin. It is an Intravenous Antimicrobial Dosed at 1.25 g Every 6 Hours and is Renally Dose-Adjusted for Patients with CrCl Less Than 60 mL/Min. I-R is Minimally Metabolized and 90% is Excreted in the Urine Unchanged
Table 4 Summary of I-R Clinical Trial Data. There are Four Clinical Trials Evaluating I-R for the Treatment of cIAIs, cUTIs, HABP, and VABP. The Two Phase 2 Trials Compared I-R at Two Different Doses to Imipenem Alone. The Primary Outcome of Favorable Clinical Response Was Similar Among All Three Treatment Groups for Both Studies for the Treatment of cIAIs and cUTIs. RESTORE-IMI 1 Compared I-R to Imipenem Plus Colistin for the Treatment of cIAIs, cUTIs, HABP, and VABP. Favorable Overall Response Was Similar Between the Groups; However Mortality Was Significantly Lower in the I-R Group. RESTORE-IMI 2 Compared I-R to Piperacillin-Tazobactam for the Treatment of HABP and VABP. Preliminary Data Suggests Day 28 All-Cause Mortality Was Lower in the I-R Group and Favorable Clinical Response Was Higher in the I-R Group
