Figures & data
Table 1 The Advantages and Disadvantages of Different Green Materials for the Synthesis of Gold Nanoparticles
Figure 3 Proposed mechanism for the reduction of gold nanoparticles where M+ is a gold (iii) ion while M0 is a zero valent gold.Citation33 “Reprinted from Journal of Advanced Research, 6, Anuradha, J, T Abbasi, and S. Abbasi, An eco-friendly method of synthesizing gold nanoparticles using an otherwise worthless weed pistia (Pistia stratiotes L.), 711-720, Copyright (2015), with permission from Elsevier.”
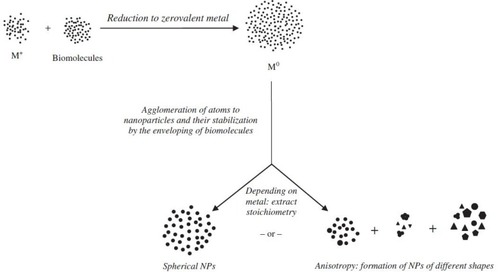
Figure 4 Some examples of plants: (A) Murraya koenigii Spreng, (B) Citrus, (C) Zingiber officinale, (D) Lonicera Japonica, (E) Garcinia mangostana and (F) abelmoschus esculentus used to synthesize gold nanoparticles.

Table 2 Green Synthesized Gold Nanoparticles Using Different Parts of Plants
Figure 6 TEM micrographs of gold nanoparticles prepared with (A) 100, (B) 200 and (C) 400 lL of a P. dactylifera extract. The corresponding histograms are represented by (A’), (B’) and (C’).Citation83 “Reprinted from Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 121, Mervat F. Zayed,Wael H. Eisa, Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity, 238-244, Copyright (2014), with permission from Elsevier.”.
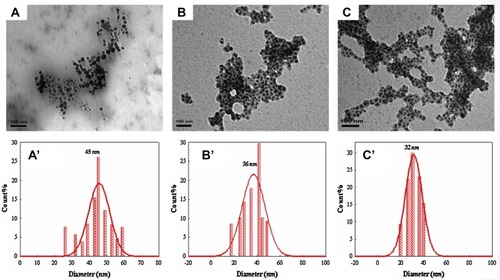
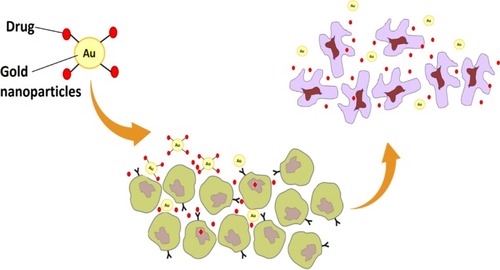
Table 3 Cancer Cells That Have Been Targeted Successfully by Gold Nanoparticles
Figure 9 (A) Fluorescence confocal images of HepG2 cells after incubation with AuNCs@ew–Hg(II) for 0, 12, 15, 21, 30 and 45 min. (B) Fluorescence confocal images of normal cells (HT22 cells) after incubation with AuNCs@ew–Hg(II) for 1.0 h. The images in the left column show the fluorescence of the AuNCs@ew added to HT22 cells; the middle column shows the DIC images of cells in bright field; and the right column shows the merging of the two previous images.Citation139 “Reprinted from Sensors and Actuators B: Chemical, 240, Lu Tian, Wenjing Zhao, Lin Li, Yaoli Tong, Guanlin Peng, Yingqi Li, Multi-talented applications for cell imaging, tumor cells recognition, patterning, staining and temperature sensing by using egg white-encapsulated gold nanoclusters, 114-124, Copyright (2017), with permission from Elsevier.”
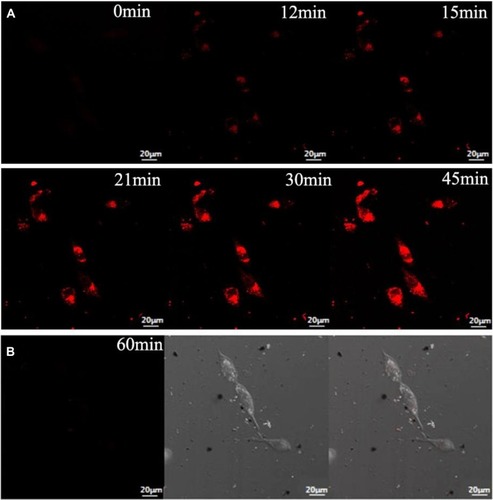
Table 4 The Shape of Gold Nanoparticles and their Applications