Figures & data
Figure 2 Determination of the antitumor activity of UA-PMs on H-22 xenograft model. The mice induced with the H-22 tumor were treated with different formulations. Outcomes measured the significant changes in the volume of tumor-induced in mice (A), changes in the weight of tumor (B), a curve showing the time of survival for the tumor-bearing mice (C), mean of the survival time (D) and weight of mice. Data are shown as the mean ± SD in each group (n=5). Significant differences were observed between treated groups and saline (*P< 0.05, **P< 0.01), treated groups and 5-FU group (#P< 0.05, ##P< 0.01), UA and UA-PMs (★P < 0.05) at the concentration of 50 mg/kg. Adapted from Zhou M, Yi Y, Liu L, et al. Polymeric micelles loading with ursolic acid enhancing antitumor effect on hepatocellular carcinoma. J Cancer. 2019;10(23):5820. Creative Commons.Citation36
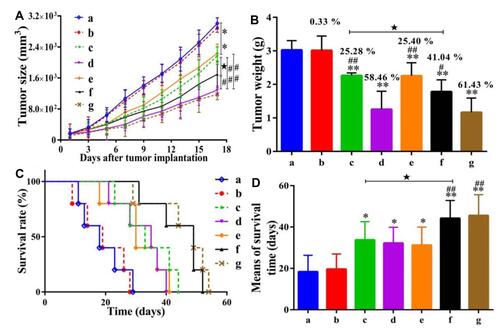
Figure 3 Heterodimeric multifunctional prodrug loaded NPs facilitated effective drug distribution to tumor and synergistic tumor chemotherapy and photodynamic therapy. (A) PET images showing the whole body of tumor-induced mice. Accumulation of NPs in tumors has been marked with white circles. (B) Quantification of the drug in tumor measured from decay-corrected PET images (n=3). (C) Ex vivo drug dissemination quantified by γ-counting of excised tissues at 48 h after the injection (n=3). (D) The volume distribution of tumor under the influence of treatment (n=5). Black arrow is a sign of intravenous injection of drugs, while red arrow reflects laser irradiation. Asterisk signs indicate the significant differences between CPT-ss-HPPH NPs and the other treatments. *p < 0.05; **p < 0.01; ***p < 0.001. *p<0.001. Adapted with permission from Zhang F, Ni Q, Jacobson O, et al. Polymeric nanoparticles with a glutathione‐sensitive heterodimeric multifunctional prodrug for in vivo drug monitoring and synergistic cancer therapy. Angewandte Chemie Int Edition. 2018;57(24):7066–7070. © 2018 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.Citation39
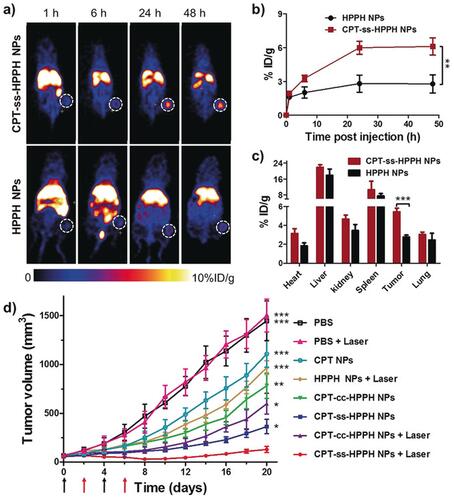
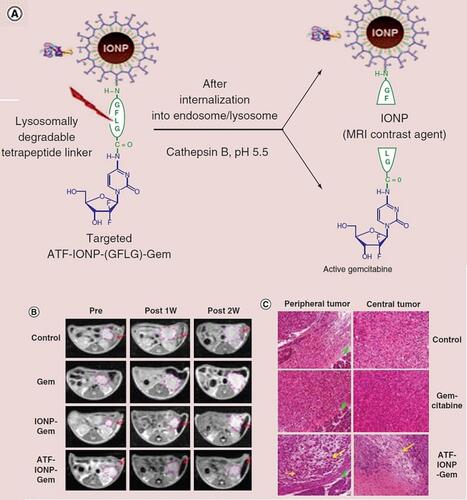
Figure 5 Magnetic resonance imaging of iron oxide nanoparticles based chemotherapy. (A) Scheme of development of gemcitabine conjugated iron oxide nanoparticles. (B) MRI observation of the therapeutic response of controlled released gemcitabine. (C) Histologic staining endorses the targeted therapeutic of pancreatic tumor. Adapted with permission from Lee GY, Qian WP, Wang L, et al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7(3):2078–2089. Copyright © 2013, American Chemical Society.Citation147