Figures & data
Figure 1 Schematic illustration of tumor and inflammation therapies by employing neutrophils, cell membrane and exosome as nanodrug delivery system, with the advantages in augmented targeting, prolonged circulation and improved biostability.
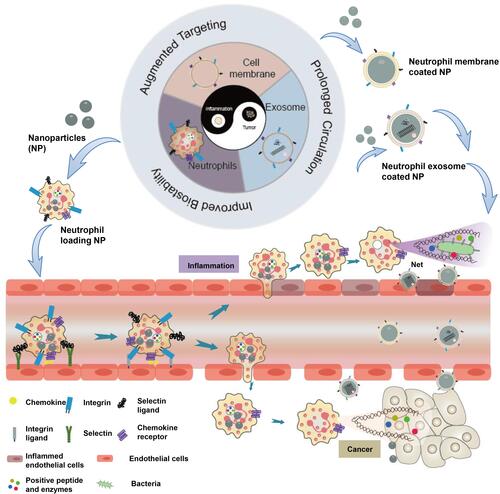
Figure 2 (A) Schematic illustration of PTX-CL/NE preparation and the procedure of administration, including intravenous injection of PEG-GNRs, conduction of PTT, and reintroduction of PTX-CL/NEs. (B) The growth profiles of HepS tumors in mice that received different treatments. The tumor sizes were monitored by a digital caliper, and the tumor volume was calculated according to the following formula: width2 × length × 0.5. (C) Representative pictures of HepS tumors that received different treatments (n=3). Reproduced with permission from Zhang L, Zhang Y, Xue YN, et al. Transforming Weakness into Strength: Photothermal-Therapy-Induced Inflammation Enhanced Cytopharmaceutical Chemotherapy as a Combination Anticancer Treatment. Adv Mater. 2019, 31, 1805936. © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.Citation70 (D) Tumor growth curve of mice with different treatments during 15 days. (E) Survival curves of Lewis tumor-bearing mice after various treatments. AuNRBR/N injected mice showed improved survival over 120 days after treatments. Reproduced from Ye B, Zhao B, Wang K, et al. Neutrophils mediated multistage nanoparticle delivery for prompting tumor photothermal therapy. J Nanobiotechnol. 2020, 18 (1), 138. Copyright © 2020, The Authors. Creative Commons CC BY.Citation72 *P < 0.05 and **P < 0.01.
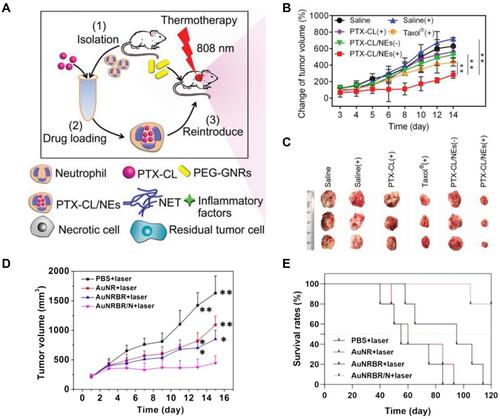
Figure 3 (A) Schematic illustration showing the chemotaxis-driven delivery of NPNs for complete eradication of tumors post-phototherapy. (B) DiD-labeled NPNs were i.v. injected into control or PTT-treated EMT6-bearing mice (n=3). At different time points, in vivo DiD fluorescent signals were observed with IVIS. (C) Two treatments of PTT plus NPNs@Pt completely eradicated tumors in all mice. Tumor growth curves during the treatments. Reproduced from Li M, Li SY, Zhou H, et al. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat Commun. 2020, 11(1), 1126. Copyright © 2020, The Authors. Creative Commons CC BY.Citation73 (D) Tumor size and (E) survival rates of the mice bearing melanoma illuminated with 660 nm laser at day 2 after the injection of vehicles, TA99, Ppa-loaded NPs, or both of TA99 and Ppa-loaded NPs. The doses of TA99 and Ppa were 40 and 2 mg kg−1, respectively. Reproduced with permission from Chu DF, Zhao Q, Yu J, Zhang FY, Zhang H, Wang ZJ. Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Adv Healthc Mater 2016, 5 (9), 1088–1093. © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.Citation74 **P < 0.01, ***P < 0.001, ****P < 0.0001.
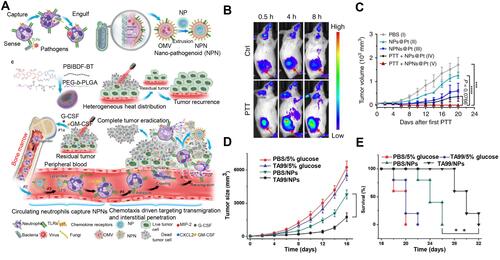
Figure 4 (A) Schematic illustration of NM-NPs loaded with carfilzomib (NM-NP-CFZ) that selectively deplete CTCs and their site of colonization. (B) Histological examination (H&E staining) of lung tissues after mice were treated with saline and low-CFZ dosage (0.5 mg/kg) of free CFZ, NP-CFZ, and NM-NP-CFZ, respectively, on days 0, 7, 14, and 21 after inoculation (n=3). Arrows show metastatic nodules. Scale bar, 5 mm. Reproduced with permission from Kang T, Zhu QQ, Wei D, et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11 (2), 1397–1411. Copyright © 2017, American Chemical Society.Citation50 (C) Relative tumor volume of 4T1-tumor-bearing mice. (D) Representative images of mice and S. aureus colonies on LB agar plates of S. aureus-infected mice at the 7th day. Reproduced with permission from Zhang C, Zhang L, Wu W, et al. Artificial Super Neutrophils for Inflammation Targeting and HClO Generation against Tumors and Infections. Adv Mater 2019, 31 (19), e1901179. © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.Citation80 *P < 0.05.
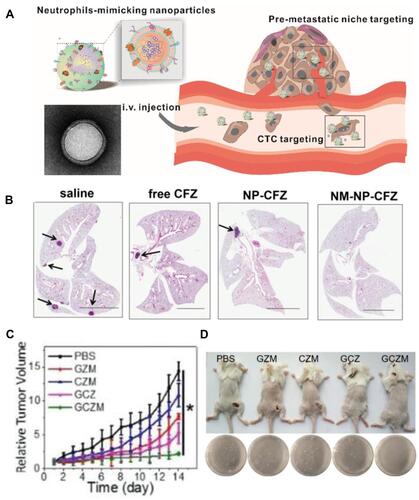
Figure 5 (A) Scheme showing NP targeting of proinflammatory neutrophils to induce their apoptosis for treatment of inflammatory diseases. (B) Cumulative DOX release from DOX-ab-BSA NPs or DOX-hyd-BSA NPs in PBS at pH 7.4, 6.5, or 5.0. (C) Percentage of apoptotic cells analyzed by flow cytometry after HL-60 cells treated with various DOX formulations. (D) Experimental design to examine the benefit of DOX-hyd-BSA NPs in cerebral I/R. OD, optical density. (E) MPO activity in brain damage tissues at 22 h after administration of PBS, free DOX, and DOX-hyd-BSA NPs. Reproduced with permission from Zhang, CY, Dong, XY, Gao, J, Lin, WJ, Liu, Z, Wang, ZJ, Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv 2019, 5 (11), eaax7964. Copyright © 2019 The Authors. Creative Commons Attribution Non Commercial License 4.0 (CC BY-NC).Citation83 (F) CLSM images of neutrophil infiltration in ischemic brain regions after treatment with saline, TNPs, and PTNPs. Neutrophils were immunostained with anti-Ly6g antibody (red) and nuclei were stained using DAPI (blue). Scale bar: 50 μm. (G) T2-weighted images of ischemic brains at 24 h in tMCAO mice treated with saline, TNPs, and PTNPs. The black curve indicates the infarct region. (H) Quantification of the infarction volumes in saline-, TNP-, and PTNP-treated tMCAO mice. Error bars indicate SD (n = 5). Reproduced with permission from Tang CM, Wang C, Zhang Y, et al. Recognition, Intervention, and Monitoring of Neutrophils in Acute Ischemic Stroke. Nano Lett 2019, 19 (7), 4470–4477. Copyright μ 2019 American Chemical Society.Citation84 *P < 0.05, and ***P < 0.001.
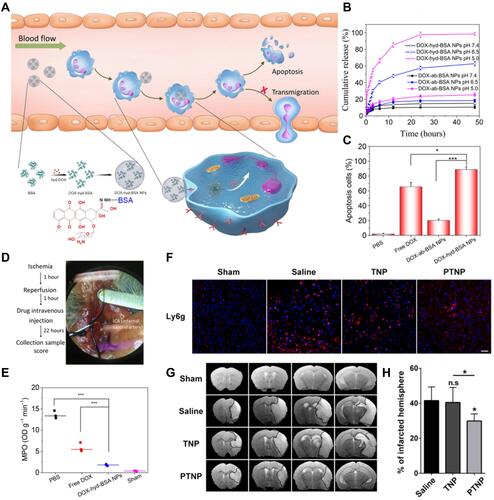
Figure 6 (A) Schematic representation of Celastrol (CLT)-loaded PEG-PLGA nanoparticles (NPs/CLT) coated with neutrophil membranes (NNPs/CLT) for the management of acute pancreatitis (AP). (B) NNPs/DiD selectively accumulated in inflamed pancreas. Ex vivo fluorescent imaging of major organs in AP rats at 1 and 3 h. Ex vivo fluorescent imaging of pancreas in AP rats at 1 and 3 h. Semiquantitative analysis of fluorescent intensity within the pancreas. Reproduced with permission from Zhou X, Cao X, Tu H, Zhang ZR, Deng L. Inflammation-Targeted Delivery of Celastrol via Neutrophil Membrane-Coated Nanoparticles in the Management of Acute Pancreatitis. Mol Pharmaceut 2019, 16 (3), 1397–1405. Copyright © 2019 American Chemical Society.Citation85 *P < 0.05 and #P < 0.05.
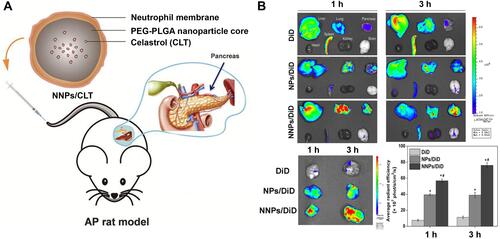
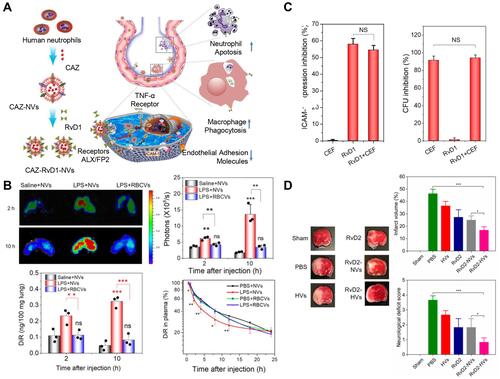
Figure 7 (A) Schematic on development of human neutrophil membrane-derived nanovesicles to target the infectious lung. (B) IVIS images of mouse lungs after LPS (at 5 mg/kg) was intratracheally administered to the mice. Quantitative analysis of fluorescent intensity of lung tissues based on the IVIS images. Nanovesicle fluorescence of lung homogenates was measured at 780 nm. Pharmacokinetics of NVs and or RBCVs in healthy mice or inflamed mice induced by intratracheal LPS administration. Reproduced with permission from Gao J, Wang S, Dong X, Leanse LG, Dai T, Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun Biol 2020, 3 (1), 680. Copyright © 2020, The Authors. Creative Commons CC BY.Citation88 (C) RvD1 and CEF inhibits ICAM-1 expression of endothelial cells and bacterial growth, respectively. (D) RvD2-nanovesicles reduce cerebral infarct sizes and protect neurological damage from ischemic stroke at 22 h postinjection of therapeutics (24 h after the MCAO surgery). Representative mouse brain sections stained with 1% of TTC in sham mice, and MCAO mice treated with PBS, HVs, RvD2, RvD2-NVs, and RvD2-HVs, respectively. Reproduced with permission from Dong XY, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. J. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13 (2), 1272–1283. Copyright © 2019 American Chemical Society.Citation90 *P<0.05, **P < 0.01, ***P < 0.001. The ns denotes nonsignificant difference.