Figures & data
Figure 1 Structural particularities of the eye. The eye can be divided into two parts of the anterior segment and the posterior segment. There are many barriers to drug delivery to the retina. Drugs cannot be easily delivered to the retina by topical administration, such as eye drops, because of the presence of tear drainage and peribulbar and choroidal blood flow. In contrast, systemically administered drugs rarely enter the retina because of the presence of the blood - aqueous barrier and the inner and outer blood-retinal barriers.
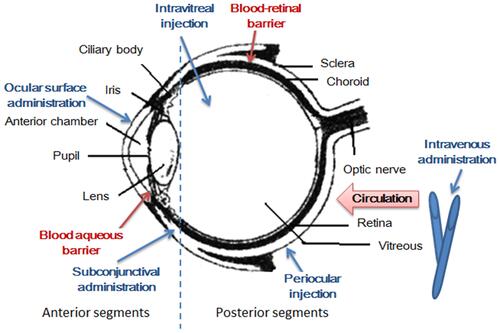
Figure 2 Electrospun nanofibers - A promising solid in-situ gel. (A) The diagram of study on electrospun nanofibers; (B) 3D diagram of the spinning solution composition and the product characteristics; (C) 3D X-ray images of the electrospun nanofibers: (black) grayscale and (color) fiber size distribution image with scale bar; (D) overlay of regular (photo) and fluorescence images of porcine eyes treated with fluorescein sodium eye drops (5 µg/mL) (left), and with pullulan-gellan gum nanofiber lens (0.0001% fluorescein sodium) (right) after different spray applications. Reprinted from Eur J Pharm Biopharm, 146, Göttel B. Electrospun nanofibers - A promising solid in-situ gelling alternative for ocular drug delivery, 125–132, Copyright 2020, with permission from Elsevier.Citation72
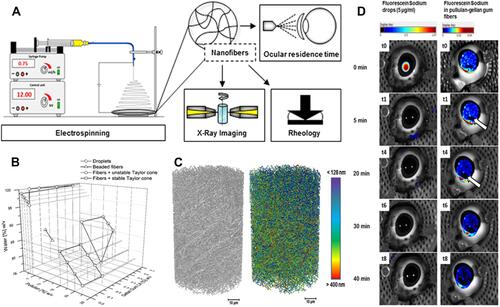
Table 1 Nanocarriers to Deliver Anti-Conjunctivitis Agents
Figure 3 Tacrolimus is a common immune modulator that has been loaded onto Poly(lactic-co-glycolic acid) nanoparticle. Hypothetical representation of the location of propylene glycol in the vesicles and its effects as a novel drug delivery system for tacrolimus. Reprinted from Colloids Surf B Biointerfaces, 157, Garg V, Suri R, Jain GK, Kohli K. Proglycosomes: a novel nano-vesicle for ocular delivery of tacrolimus, 40–47, Copyright 2017, with permission from Elsevier.Citation85
Abbreviation: PNVs, Proglycosomes nano-vesicles.
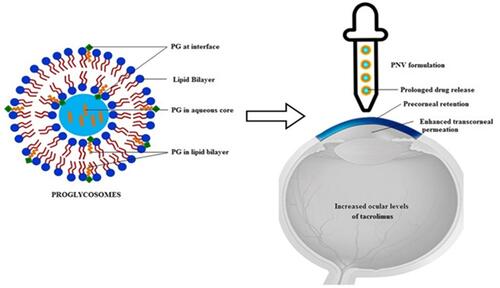
Table 2 Nanocarriers to Deliver Anti-Dry Eye Agents
Table 3 Nanocarriers to Deliver Anti-Glaucoma Agents
Figure 4 Cerium (III) chloride (CeCl3)-loaded mesoporous silica (CeCl3@mSiO2) nanoparticles. A type of cerium (III) chloride (CeCl3)-loaded mesoporous silica (CeCl3@mSiO2) nanoparticles designed and to prevent the formation of diabetic cataract in vitro and in vivo using a well-established rat model of streptozotocin-induced diabetes. (A) Schematic diagram of CeCl3@mSiO2 nanoparticle preparation; (B) The morphology of the mSiO2 nanoparticles under transmission electron microscopy (spherical, uniform and monodisperse morphology with an average diameter of 85 nm); (C) A line graph, showing the time course of changes in the cataract degrees in different groups of animals; (D) various degrees of cataract and representative images of H&E-stained sections of the lens tissue in experimentally induced diabetic animals 8 weeks after treatment with vehicle or 10 and 20 mg/kg CeCl3@mSiO2 nanoparticles, respectively. Reprinted from Nanomed, 13, Yang J, Gong X, Fang L, et al. Potential of CeCl3@mSiO2 nanoparticles in alleviating diabetic cataract development and progression, 147–1155, Copyright 2017, with permission from Elsevier.Citation110
Table 4 Nanocarriers to Deliver Anti-Age-Related Macular Degeneration Agents
Table 5 Nanocarriers to Deliver Anti-Uveitis Agents
Table 6 Nanocarriers to Deliver Anti-VEGF Agents
