Figures & data
Figure 1 Schematic illustration of the cause of acute liver injury and ROS-scavenging nanozymes for alleviation of acute liver injury.
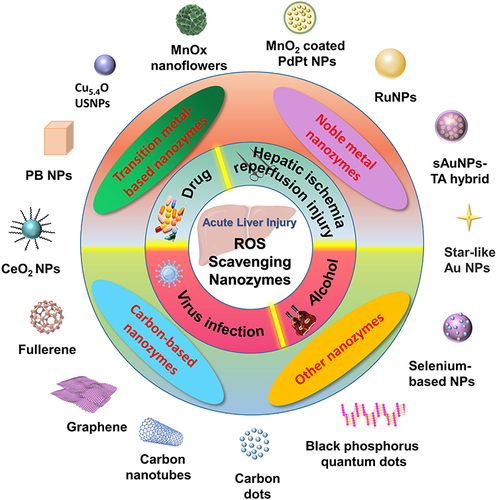
Table 1 Summary of Mechanisms for Different Types of Acute Liver Injury
Table 2 Summary of ROS-Scavenging Nanozymes for the Alleviation of Acute Liver Injury
Figure 2 Ce-based nanozymes (CeNZs) for ALI alleviation. (A) Schematic illustration of preventing hepatic ischemia-reperfusion injury by ceria NPs. Reprinted from Ni D, Wei H, Chen W, et al. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater. 2019;31:1902956. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.Citation35 (B) Schematic mechanism of ceria nanozymes for drug-induced liver injury therapy by dual detoxification and inflammatory regulation. Reprinted from Nano Today, 35, Li F, Qiu Y, Xia F, et al. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. 100925, copyright 2020, with permission from Elsevier.Citation58 (C) Schematic illustration of the preparation of mesoporous hollow manganese doped ceria nanoparticle and application for effectively prevention of hepatic ischemia reperfusion injury. Reprinted from Dove Medical Press, Si PR, Lei JX, Yang C, et al. Mesoporous hollow manganese doped ceria nanoparticle for effectively prevention of hepatic ischemia reperfusion injury. Int J Nanomedicine. 2023;18:2225–2238.Citation71
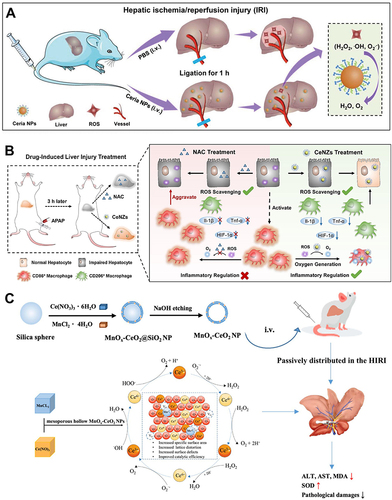
Figure 3 Fe-based nanozymes for ALI alleviation. (A) Schematic illustrations of PB nanozymes preventing anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation. Bai H, Kong F, Feng K, et al. Prussian blue nanozymes prevent anthracycline-induced liver injury by attenuating oxidative stress and regulating inflammation. ACS Appl Mater Interfaces. 2021;13:42382–42395. Copyright © 2021 American Chemical Society.Citation61 (B) Schematic diagram of the mechanisms of PB scavengers protect against hepatic ischemia reperfusion injury. Huang YX, Xu QY, Zhang J, et al. Prussian blue scavenger ameliorates hepatic ischemia-reperfusion injury by inhibiting inflammation and reducing oxidative stress. Front Immunol. 2022;13:891351.Citation77 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (C) Schematic illustrations of PB nanozyme impregnated mesenchymal stem cells for hepatic ischemia-reperfusion injury alleviation. Reprinted from Sahu A, Jeon J, Lee MS, et al. Nanozyme impregnated mesenchymal stem cells for hepatic ischemia-reperfusion injury alleviation. ACS Appl Mater Interfaces. 2021;13:25649–25662. Copyright © 2021 American Chemical Society.Citation76 (D) Schematic illustrations of PB nanozymes preventing APAP-induced liver injury by scavenging ROS, relieving oxidative stress, and regulating the inflammatory response. Feng Q, Xu H, Pan X, et al. Antioxidation and anti-inflammatory activity of Prussian blue nanozymes to alleviate acetaminophen-induced acute liver injury. ACS Appl Nano Mater. 2023;6:8468–8481. Copyright © 2023 American Chemical Society.Citation60
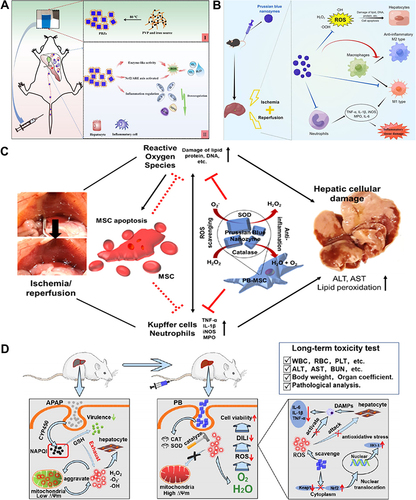
Figure 4 Cu-based nanozymes for ALI alleviation. (A) Schematic illustration of Cu5.4O ultrasmall nanoparticles (USNPs) as a broad-spectrum ROS scavenging agent in the treatment of ROS-related diseases, including ALI, acute kidney injury and diabetic wound healing. Liu T, Xiao B, Xiang F, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788.Citation13 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (B) Schematic illustration of the HLCs/Cu NZs@fiber/dECM for CCl4-induced acute liver failure through the effects of ROS elimination, angiogenesis promotion, anti-inflammation, hepatocyte-related functions, and facilitating liver regeneration. Reprinted from Bioact Mater, 28, Jin Y, Zhang J, Xu Y, et al. Stem cell-derived hepatocyte therapy using versatile biomimetic nanozyme incorporated nanofiber-reinforced decellularized extracellular matrix hydrogels for the treatment of acute liver failure. 112–131, Copyright 2023.Citation62 This is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
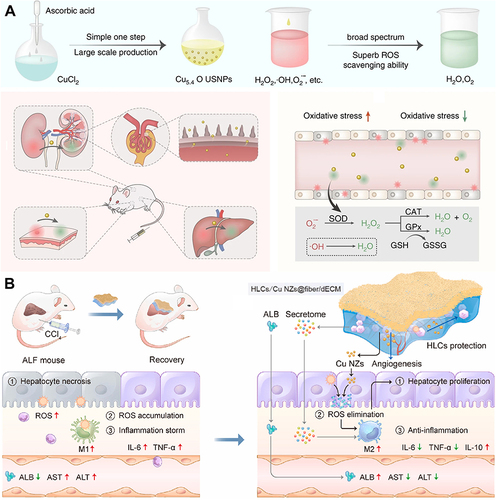
Figure 5 Mn-based nanozymes for ALI alleviation. (A) Schematic illustration of the preparation of TMSN@PM. (B) TEM image of TMSN@PM. (C) Schematic illustration of the in vivo MRI-guided real-time monitoring of the treatment process of inflammatory diseases using TMSN@PM. Reproduced with permission from Li X, Liu Y, Qi X, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34:e2109004. © 2022 Wiley-VCH GmbH.Citation80
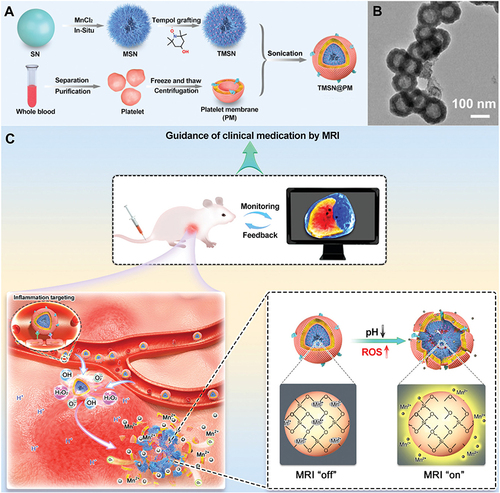
Figure 6 Other transition metal-based nanozymes for ALI alleviation. (A) FESEM image of WS2-PVP nanoflowers. (B) CAT-like activity, SOD-like activity and GPx-like activity of WS2-PVP nanoflowers. (C) Schematic diagram of ALI treatment with WS2-PVP nanoflowers. Reprinted from J Colloid Interf Sci, 625, Xu H, Zhang ZR, Zhang LY, et al. Tungsten disulfide nanoflowers with multi-nanoenzyme activities for the treatment of acute liver injury. 544–554, Copyright 2022, with permission from Elsevier.Citation63
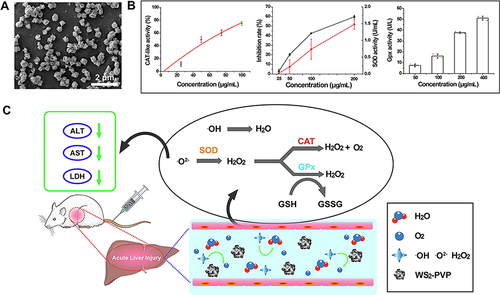
Figure 7 Noble metal nanozymes for ALI alleviation. (A) Schematic illustration of tuning the size of RuNPs to boost their antioxidant activity and the application for sRuNP for highly efficient ALI therapy. Reprinted from Xia F, Hu X, Zhang B, et al. Ultrasmall ruthenium nanoparticles with boosted antioxidant activity upregulate regulatory T cells for highly efficient liver injury therapy. Small. 2022;18:2201558. © 2022 Wiley-VCH GmbH.Citation102 (B) Schematic illustration of synthesis of Pt@CNDs with cascade superoxide dismutase-catalase activities and applications in eliminating intracellular ROS. Reprinted from Nano Today, 49, Zhang Y, Gao W, Ma Y, et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. 101768, Copyright 2023, with permission from Elsevier.Citation103 (C) Schematic representation of SAuPTB nanozymes relieves APAP-induced ALI by attenuating ROS and regulating inflammation. Reprinted from Zhou C, Zhang L, Xu Z, et al. Self-Propelled Ultrasmall AuNPs-tannic acid hybrid nanozyme with ROS-scavenging and anti-inflammatory activity for drug-induced liver injury alleviation. Small. 2023;19:2206408. Copyright © 2023 Wiley-VCH GmbH.Citation106
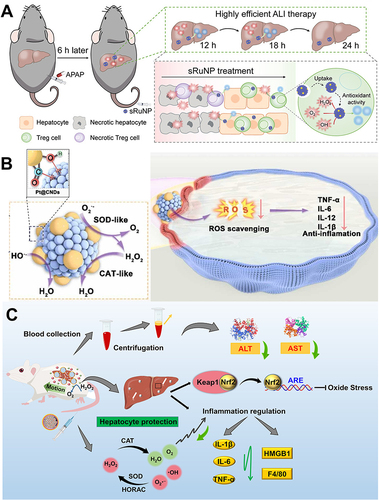
Figure 8 Carbon-based nanozymes for ALI alleviation. (A) Schematic illustration of esterase-responsive CD-Dex with ROS elimination and inflammation suppression capabilities for liver fibrosis therapy. Reprinted from Xu YC, Chen J, Jiang W, et al. Multiplexing nanodrug ameliorates liver fibrosis via ROS elimination and inflammation suppression. Small. 2022;18:2102848. Copyright © 2021 Wiley-VCH GmbH.Citation113 (B) Schematic illustration of [70]/[60] fullerenols against oxidative injury induced by reduplicative chemotherapy. Reprinted from Zhou Y, Li J, Ma HJ, et al. Biocompatible [60] / [70] fullerenols: potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mater Interfaces. 2017;9:35539–35547. Copyright © 2017 American Chemical Society.Citation111 (C) Schematic illustration of the mechanism of self-assembled selenium-doped carbon quantum dots as antioxidants for HIRI management. Bai B, Qi S, Yang K, et al. Self-assembly of selenium-doped carbon quantum dots as antioxidants for hepatic ischemia-reperfusion injury management. Small. 2023;19:2300217. Copyright © 2023 Wiley-VCH GmbH.Citation115 (D) Schematic illustration of nitrogen-doped carbon dots (CNDs) for reactive oxygen species scavenging on HIRI. Reprinted from Chen D, Wang CQ, Yu HJ, et al. Nitrogen-doped carbon dots with oxidation stress protective effects for reactive oxygen species scavenging on hepatic ischemia-reperfusion injury. ACS Appl Nano Mater. 2023. Copyright © 2023 American Chemical Society.Citation114 (E) Schematic of carbohydrate-derived nanoparticles (C-NPs) for Hepatic ischemia-reperfusion injury treatment. Reprinted from Long Y, Wei H, Li J, et al. Prevention of hepatic ischemia-reperfusion injury by carbohydrate-derived nanoantioxidants. Nano Lett. 2020;20:6510–6519. Copyright © 2020 American Chemical Society.Citation112
![Figure 8 Carbon-based nanozymes for ALI alleviation. (A) Schematic illustration of esterase-responsive CD-Dex with ROS elimination and inflammation suppression capabilities for liver fibrosis therapy. Reprinted from Xu YC, Chen J, Jiang W, et al. Multiplexing nanodrug ameliorates liver fibrosis via ROS elimination and inflammation suppression. Small. 2022;18:2102848. Copyright © 2021 Wiley-VCH GmbH.Citation113 (B) Schematic illustration of [70]/[60] fullerenols against oxidative injury induced by reduplicative chemotherapy. Reprinted from Zhou Y, Li J, Ma HJ, et al. Biocompatible [60] / [70] fullerenols: potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mater Interfaces. 2017;9:35539–35547. Copyright © 2017 American Chemical Society.Citation111 (C) Schematic illustration of the mechanism of self-assembled selenium-doped carbon quantum dots as antioxidants for HIRI management. Bai B, Qi S, Yang K, et al. Self-assembly of selenium-doped carbon quantum dots as antioxidants for hepatic ischemia-reperfusion injury management. Small. 2023;19:2300217. Copyright © 2023 Wiley-VCH GmbH.Citation115 (D) Schematic illustration of nitrogen-doped carbon dots (CNDs) for reactive oxygen species scavenging on HIRI. Reprinted from Chen D, Wang CQ, Yu HJ, et al. Nitrogen-doped carbon dots with oxidation stress protective effects for reactive oxygen species scavenging on hepatic ischemia-reperfusion injury. ACS Appl Nano Mater. 2023. Copyright © 2023 American Chemical Society.Citation114 (E) Schematic of carbohydrate-derived nanoparticles (C-NPs) for Hepatic ischemia-reperfusion injury treatment. Reprinted from Long Y, Wei H, Li J, et al. Prevention of hepatic ischemia-reperfusion injury by carbohydrate-derived nanoantioxidants. Nano Lett. 2020;20:6510–6519. Copyright © 2020 American Chemical Society.Citation112](/cms/asset/1bcf74e4-dcc8-4231-aa40-f7f863e17052/dijn_a_12299243_f0008_c.jpg)
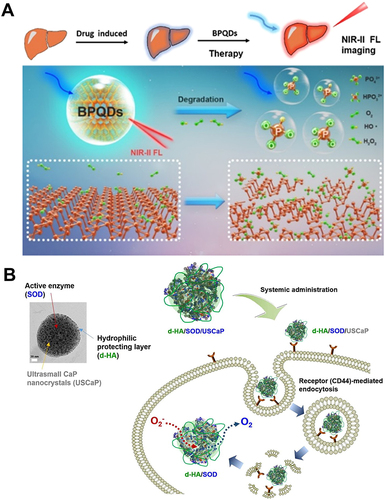
Figure 9 Carbon-based nanozymes for ALI alleviation. (A) Schematic illustration of NIR-II fluorescent biodegradable black phosphorus quantum dots (BPQDs) for precise acute liver injury imaging and therapy. Ge X, Su L, Yang L, et al. NIR-II fluorescent biodegradable nanoprobes for precise acute kidney/liver injury imaging and therapy. Anal Chem. 2021;93:13893–13903. Copyright © 2021 American Chemical Society.Citation116 (B) Schematic illustration of targeted cellular delivery of robust d-HA/SOD/USCaP enzyme nanoparticles for the treatment of drug-induced liver injury. Reprinted from Acta Biomater, 81, Lee MS, Kim NW, Lee JE, et al. Targeted cellular delivery of robust enzyme nanoparticles for the treatment of drug-induced hepatotoxicity and liver injury. 231–241, Copyright 2018, with permission from Elsevier.Citation117