Figures & data
Figure 1 Design of genetically engineered drug carriers.
Notes: The field of biological nanomedicine (aka “BioNano”) is emerging at the intersections between genetically engineered biomaterials, nano-assembly, and protein polymers. At intersection 1, nanomedicines are being developed from protein polymers (eg, ELP, SLP, and SELP). At intersection 2, protein-based materials (eg, viral capsids and vault proteins) are being developed as platforms for assembly of nanostructures. At intersection 3, proteins that avoid structure formation (eg, intrinsically disordered proteins and XTEN fusion proteins) are being explored for their ability to alter biodistribution and efficacy.
Adapted with permission from Galaway FA, Stockley PG. MS2 viruslike particles: a robust, semisynthetic targeted drug delivery platform. Mol Pharm. 2013;10(1): 59–68.Citation55 Copyright 2013 American Chemical Society, and Buehler DC, Toso DB, Kickhoefer VA, Zhou ZH, Rome LH. Vaults engi neered for hydrophobic drug delivery. Small. 2011;7(10):1432–1439.Citation7 Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Abbreviations: ELP, elastin-like polypeptide; SLP, silk-like polypeptide; XTEN, extended recombinant polypeptide.
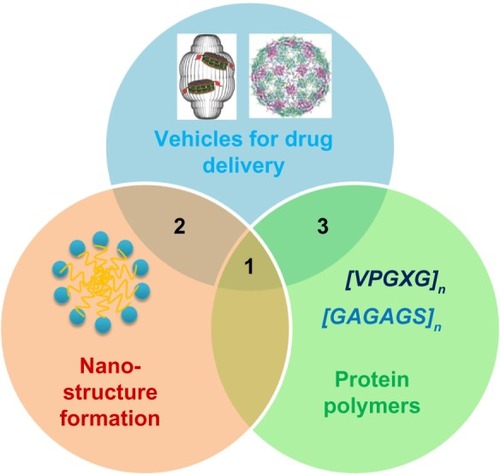
Figure 2 TEM of ELP nanoparticles. Diblock copolymers composed of ELPs with various guest residues assemble micelles.
Notes: (A) TEM image of A96I96, which has a hydrophilic (Xaa = Ala, n=96, N-terminus) and a hydrophobic (Xaa = Ile, n=96, C-terminus) block. Scale bar 50 nm. From Janib SM, Liu S, Park R, et al. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integr Biol (Camb). 2013;5(1):183–194.Citation23 Reproduced by permission of The Royal Society of Chemistry. (B) TEM image of ELP I96S96, which has a hydrophilic (Xaa = Ser, n=96, C-terminus) and a hydrophobic (Xaa = Ile, n=96, N-terminus) block. Scale bar 200 nm. From Janib SM, Pastuszka MF, Aluri S, et al. A quantitative recipe for engineering protein polymer nanoparticles. Polym Chem. 2014;5(5):1614–1625.Citation4 Reproduced by permission of The Royal Society of Chemistry. (C) Cryo-TEM image of ELP E50I60, which has a hydrophilic (Xaa = Val:Glu [4:1], n=50, N-terminus) and a hydrophobic (Xaa = Ile, n=60, C-terminus) block. Scale bar 100 nm. Reproduced with permission from García-Arévalo C, Bermejo-Martín JF, Rico L, et al. Immunomodulatory nanoparticles from elastin-like recombinamers: single-molecules for tuberculosis vaccine development. Mol Pharm. 2013;10(2):586–597.Citation34 Copyright 2013 American Chemical Society. (D) Cryo-TEM image of ELP-96/90, which has a hydrophilic (Xaa = Val:Ala:Gly [1:8:7], n=96, N-terminus) and a hydrophobic (Xaa = Val, n=90, C-terminus) block. Scale bar 20 nm. Reprinted with permission from Dreher MR, Simnick AJ, Fischer K, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130(2):687–694.Citation33 Copyright 2008 American Chemical Society.
Abbreviations: ELP, elastin-like polypeptide; TEM, transmission electron microscopy; cryo-TEM, cryogenic transmission electron microscopy.
![Figure 2 TEM of ELP nanoparticles. Diblock copolymers composed of ELPs with various guest residues assemble micelles.Notes: (A) TEM image of A96I96, which has a hydrophilic (Xaa = Ala, n=96, N-terminus) and a hydrophobic (Xaa = Ile, n=96, C-terminus) block. Scale bar 50 nm. From Janib SM, Liu S, Park R, et al. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integr Biol (Camb). 2013;5(1):183–194.Citation23 Reproduced by permission of The Royal Society of Chemistry. (B) TEM image of ELP I96S96, which has a hydrophilic (Xaa = Ser, n=96, C-terminus) and a hydrophobic (Xaa = Ile, n=96, N-terminus) block. Scale bar 200 nm. From Janib SM, Pastuszka MF, Aluri S, et al. A quantitative recipe for engineering protein polymer nanoparticles. Polym Chem. 2014;5(5):1614–1625.Citation4 Reproduced by permission of The Royal Society of Chemistry. (C) Cryo-TEM image of ELP E50I60, which has a hydrophilic (Xaa = Val:Glu [4:1], n=50, N-terminus) and a hydrophobic (Xaa = Ile, n=60, C-terminus) block. Scale bar 100 nm. Reproduced with permission from García-Arévalo C, Bermejo-Martín JF, Rico L, et al. Immunomodulatory nanoparticles from elastin-like recombinamers: single-molecules for tuberculosis vaccine development. Mol Pharm. 2013;10(2):586–597.Citation34 Copyright 2013 American Chemical Society. (D) Cryo-TEM image of ELP-96/90, which has a hydrophilic (Xaa = Val:Ala:Gly [1:8:7], n=96, N-terminus) and a hydrophobic (Xaa = Val, n=90, C-terminus) block. Scale bar 20 nm. Reprinted with permission from Dreher MR, Simnick AJ, Fischer K, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130(2):687–694.Citation33 Copyright 2008 American Chemical Society.Abbreviations: ELP, elastin-like polypeptide; TEM, transmission electron microscopy; cryo-TEM, cryogenic transmission electron microscopy.](/cms/asset/a510aca2-f86d-40ef-a9a8-0e28fd5e770c/dijn_a_53886_f0002_b.jpg)
Figure 3 Rapa encapsulated by FKBP-decorated nanoparticles has both anticancer and immunosuppressive efficacy.
Notes: (A) ELP nanoparticles fused genetically to the cognate receptor of Rapamycin-FKBP can specifically carry the drug with high avidity. (B) FSI significantly prolongs drug release compared with plain ELP (SI). (C) FSI-Rapa has lower cytotoxicity and greater antitumor efficacy than free Rapa in an MDA-MB-468 breast tumor xenograft mouse model. Compared with free Rapa group, which showed severe cytotoxicity (15% bodyweight loss by day 23), no obvious systemic cytotoxicity was observed in the FSI-Rapa group. (A), (B), (C) Reproduced from J Control Release,171(3), Shi P, Aluri S, Lin YA, et al. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo, 330–338,Citation42 Copyright 2013, with permission from Elsevier. (D) FSI-Rapa suppresses transcription and expression of the protease cathepsin-S (CATS), a biomarker of lacrimal gland autoimmune dacryoadenitis, better than free Rapa in a mouse model of Sjögren’s syndrome. Reproduced from J Control Release, 171(3), Shah M, Edman MC, Janga SR, et al, A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren’s syndrome, 269–279,Citation43 Copyright 2013 with permission from Elsevier. (E) The blood half-lives of four ELPs estimated by pharmacokinetic modeling in mice based on micro-PET imaging. ***indicates a P-value of <0.001; **indicates a P-value of <0.01 by one-way ANOVA with Tukey’s multiple comparison test. From Janib SM, Liu S, Park R, et al. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integr Biol (Camb). 2013;5(1):183–194.Citation23 Reproduced by permission of The Royal Society of Chemistry.
Abbreviations: CMT, critical micelle temperature; ELP, elastin-like polypeptide; FKBP, FK506-binding protein; FSI, FKBP-ELP; PBS, phosphate buffered saline; PET, positron emission tomography; Rapa, rapamycin; SI, ELP S48I48.
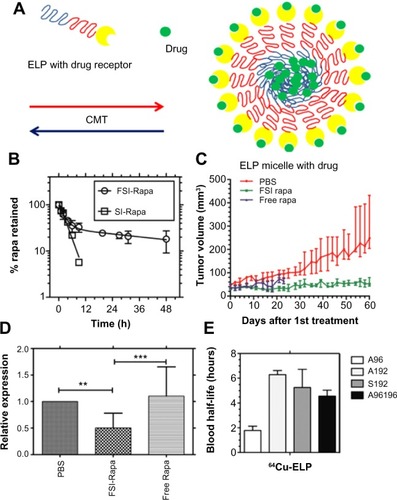
Figure 4 Silk-elastin-like protein polymers with different ratios of silk to elastin self-assemble into various nanostructures.
Notes: (A) SELP constructs SE8Y, S2E8Y, and S4E8Y, which contain varying ratios of the silk-to-elastin blocks in each monomer repeat. (B and C) Atomic force microscopy images present the nanostructures self-assembled from SE8Y, S2E8Y, and S4E8Y at 60°C (B) and 20°C (C).
Adapted with permission from Xia XX, Xu Q, Hu X, Qin G, Kaplan DL. Tunable self-assembly of genetically engineered silk – elastin-like protein polymers. Biomacromolecules. 2011;12(11):3844–3850.Citation28 Copyright 2011 American Chemical Society.
Abbreviation: SELP, silk-elastin-like polypeptide.
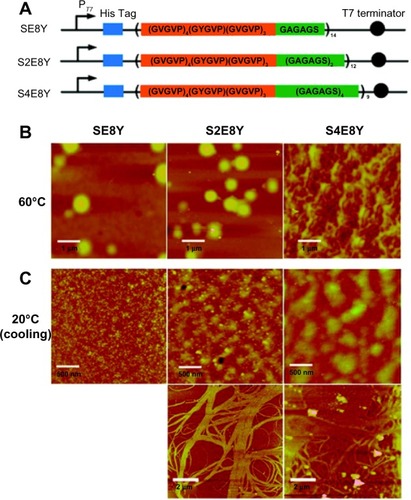
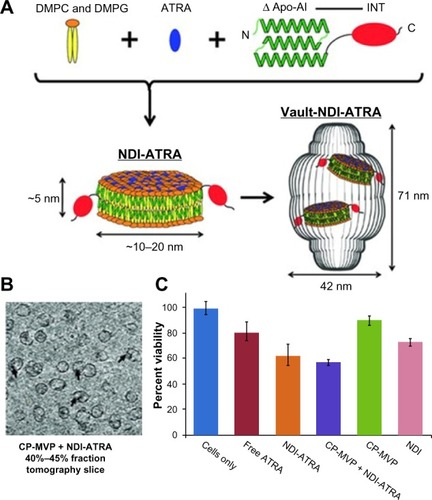
Figure 5 Vault protein engineered for hydrophobic drug delivery.
Notes: (A) Schematic diagram representing NDI-ATRA formation and encapsulation by the vault nanoparticle. Components are not drawn to scale. (B) CP-MVP + NDI-ATRA electron microscopic tomography slice showing NDI-ATRA vault packaging. (C) HepG2 cell viability assay. NDI-ATRA and CP-MVP + NDI-ATRA both display increased toxicity than free ATRA over the course of 120 hours.
Adapted with permission from Buehler DC, Toso DB, Kickhoefer VA, Zhou ZH, Rome LH. Vaults engi neered for hydrophobic drug delivery. Small. 2011;7(10):1432–1439.Citation7 Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Abbreviations: ΔApo-AI, a truncated form of apolipoprotein-AI (Apo-AI, amino acids 44–200); ATRA, all-trans retinoic acid; CP-MVP, the purified vaults; DMPC, dimyristoylphosphatidylcholine; DMPG, dimyristoylphosphatidylglycerol; INT, vault-targeting domain; NDI, nanodisk-INT complex.