Figures & data
Figure 1 Synthesis route for end functional PLA showing a) zinc proline at 180°C, b) succinic anhydride, TEA, DMAP at room temperature.
Abbreviations: COOH-PLA, carboxyl-terminated PLA; DMAP, 4-dimethylaminopyridine; OH-PLA, OH-terminated polylactide; PLA, polylactide; TEA, triethylamine.
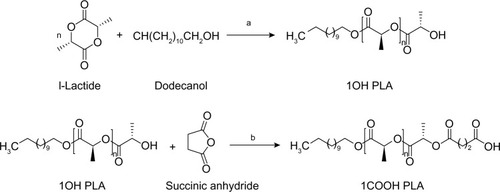
Figure 2 1H NMR measurement of 7-(4-(2-chloroacetyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylic acid.
Abbreviations: DSMO, dimethyl sulfoxide; 1H NMR, proton nuclear magnetic resonance.
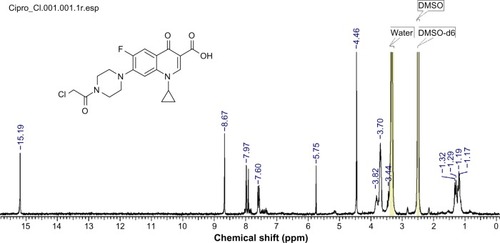
Figure 3 1H NMR measurement of ciprofloxacin-conjugated PLA in DMSO-d6.
Note: The red bond indicates the new conjugation bond formation between PLA and Cp1.
Abbreviations: Cp1, 7-(4-(2-chloroacetyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylic acid; DSMO, dimethyl sulfoxide; 1H NMR, proton nuclear magnetic resonance; PLA, polylactide; TMS, chloromethyl trimethylsilane.
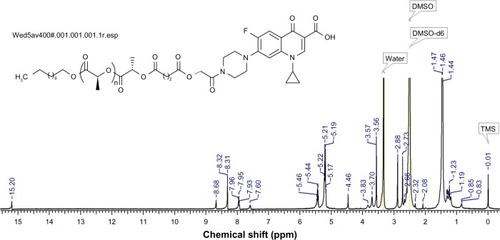
Figure 4 Synthesis of ciprofloxacin derivative and conjugation with end functional PLA a) TEA, ClCH2COCl, CH2Cl2, 0°C– room temperature; b) K2CO3, DMF, 100°C.
Abbreviations: Cp, ciprofloxacin; Cp1, 7-(4-(2-chloroacetyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylic acid; COOH-PLA, carboxyl-terminated PLA; DMF, N,N-dimethylformamide; PLA, polylactide; TEA, triethylamine.
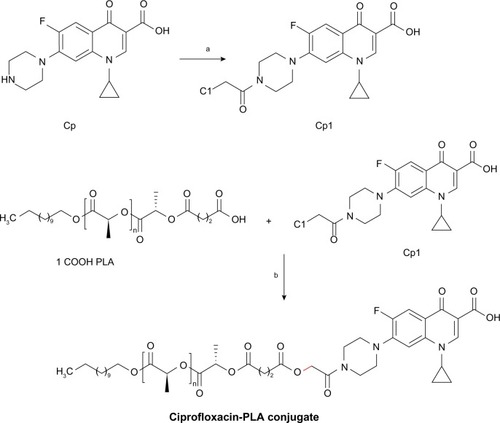
Figure 5 MALDI-TOF MS for the hydroxyl-, carboxyl- and ciprofloxacin-terminated PLA.
Notes: MALDI-TOF/TOF spectra of hydroxyl-terminated PLA (1OH-PLA) (A); MALDI-TOF/TOF spectra of carboxyl-terminated PLA (1COOH-PLA) (B); and MALDI-TOF/TOF spectra of ciprofloxacin conjugated PLA (PLA-CP) (C).
Abbreviations: MALDI-TOF MS, matrix-assisted laser desorption/ionization-time of flight mass spectrometry; PLA, polylactide.
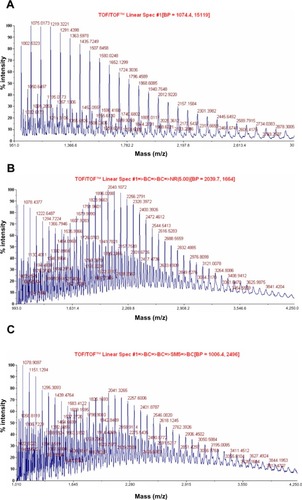
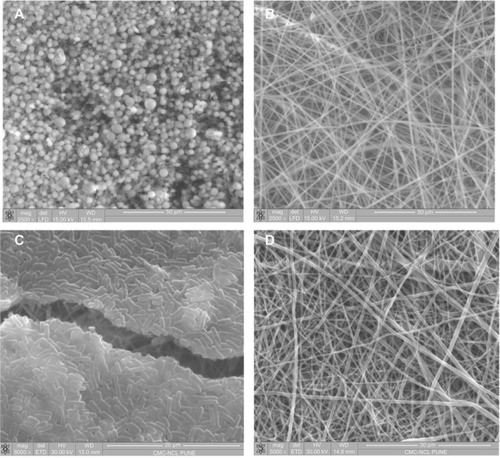
Figure 6 SEM images of PLA nanofibers (A), PLA-CP (0.003 mol) nanofibers (B), PLA-CP (0.015 mol) nanofibers (C), and PLA-CP nanofiber after 1 hour heat treatment (D).
Abbreviations: Cp1, 7-(4-(2-chloroacetyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylic acid; PLA, polylactide; PLA-CP, ciprofloxacin-conjugated PLA; SEM, scanning electron microscopy.
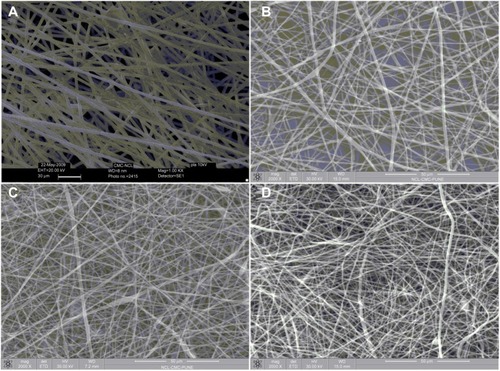
Figure 7 SEM images of PLA nanofibers with nano pores.
Abbreviations: PLA, polylactide; SEM, scanning electron microscopy.
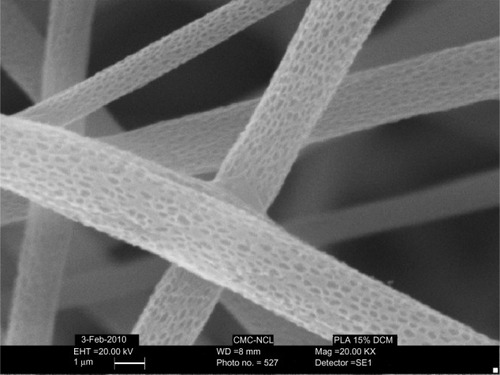
Figure 8 Antibacterial effect of nonwoven nanomats containing different concentrations of ciprofloxacin against Staphylococcus aureus.
Notes: Control PLA nanofiber mat a); PLA nanofiber mat (0.15 μg/mL) b); PLA nanofiber mat (0.3 μg/mL) c); PLA nanofiber mat (0.45 μg/mL) d); and PLA nanofiber mat (0.55 μg/mL) e).
Abbreviations: PLA, polylactide; SD, standard deviation.
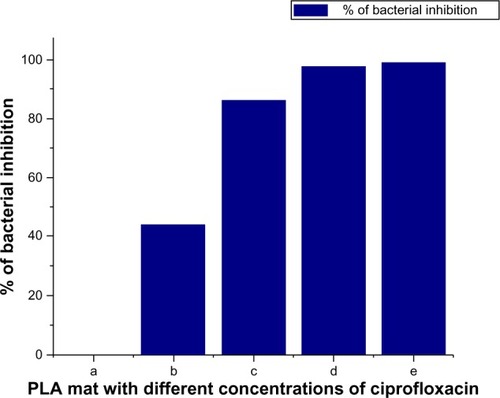
Figure 9 Antibacterial performances of PLA and ciprofloxacin-conjugated PLA nanofibers.
Notes: Staphylococcus aureus on PLA nonwoven electrospun mat (A); Staphylococcus aureus on PLA-CP nonwoven electrospun mat (B); Escherichia coli on PLA nonwoven electrospun mat (C); Escherichia coli on PLA-CP nonwoven electrospun mat (D).
Abbreviations: PLA, polylactide; PLA-CP, ciprofloxacin-conjugated PLA.
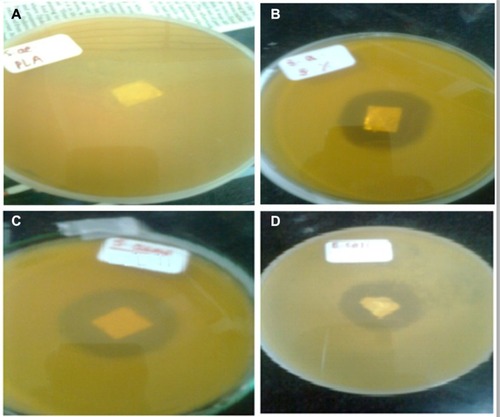
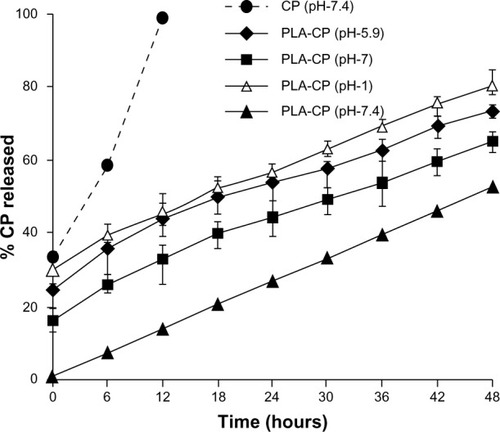
Table 1 Ciprofloxacin release in the presence of Escherichia coli at various times
Figure 10 SEM images of PLA and PLA-CP nanofiber mats with bacteria.
Notes: PLA-CP mat with Staphylococcus aureus (A); PLA-CP mat without Staphylococcus aureus. (B); PLA-CP mat with Escherichia coli (C); PLA-CP-mat without Escherichia coli (D).
Abbreviations: PLA, polylactide; PLA-CP, ciprofloxacin-conjugated PLA; SEM, scanning electron microscope.