Figures & data
Table 1 Composition of hydrogels
Figure 1 Viscosity as a function of shear rate of (A) chitosan hydrogels containing nanocapsules and (B) chitosan hydrogels containing ethanol and a commercial formulation of capsaicinoids.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids; ET, ethanolic solution; Commercial, commercial formulation.
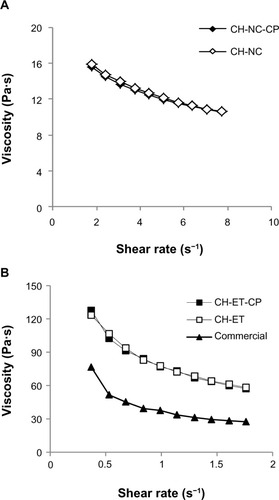
Figure 2 Size distribution analyses of chitosan hydrogels containing nanocapsules.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids.
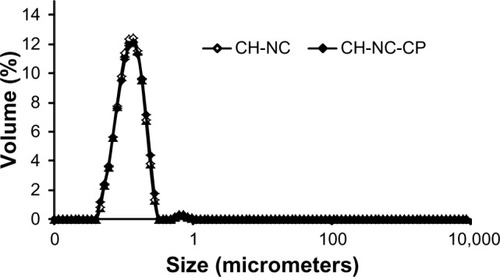
Figure 3 Skin erythema measured by electronic probe. The results indicate a ratio between the initial values (before application of formulation) and the final value at every measuring time point.
Notes: Significant differences observed (P<0.05): *CH-ET-CP versus no formulation; **commercial formulation versus no formulation.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids; ET, ethanolic solution; Commercial, commercial formulation.
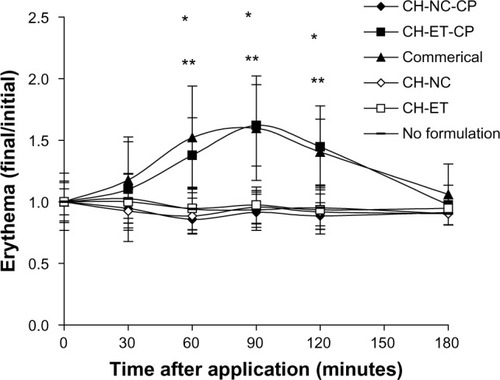
Figure 4 Skin erythema measured by a visual scale.
Notes: Significant differences observed (P<0.05): *CH-ET-CP versus no formulation; **commercial formulation versus no formulation.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids; ET, ethanolic solution; Commercial, commercial formulation.
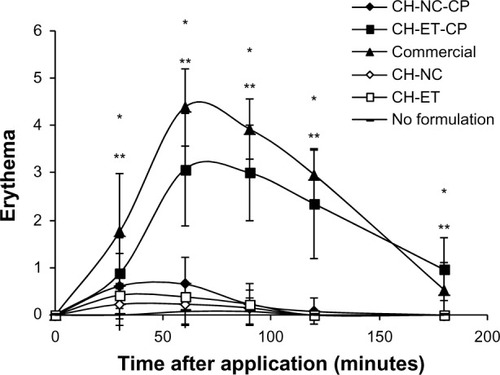
Figure 5 Arm of a volunteer 90 minutes after application of the formulations. The photograph is representative of the entire experiment.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids; ET, ethanolic solution; Commercial, commercial formulation.
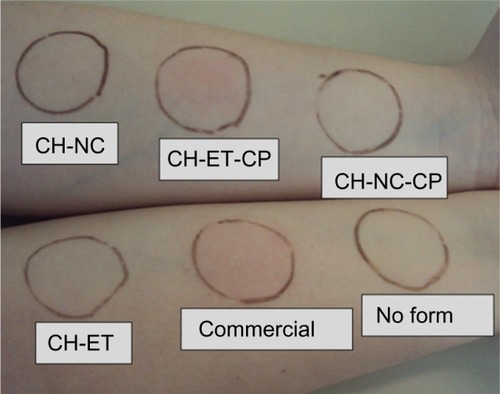
Figure 6 Skin pH (A) and transepidermal water loss (B) measured by electronic probe. The results indicate a ratio between the initial values (before application of formulation) and the final value at every measuring time point.
Note: No significant differences (P<0.05) are observed between areas with and without application of the formulations.
Abbreviations: CH, chitosan gel; NC, nanocapsules; CP, capsaicinoids; ET, ethanolic solution; Commercial, commercial formulation.
Table 2 Sensory analyses of skin irritation
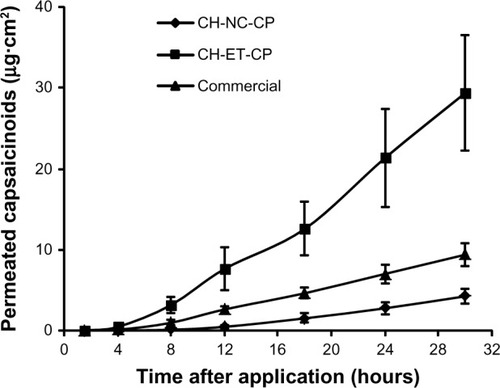
Table 3 Skin permeation parameters of capsaicinoids from different semi-solid formulations