Figures & data
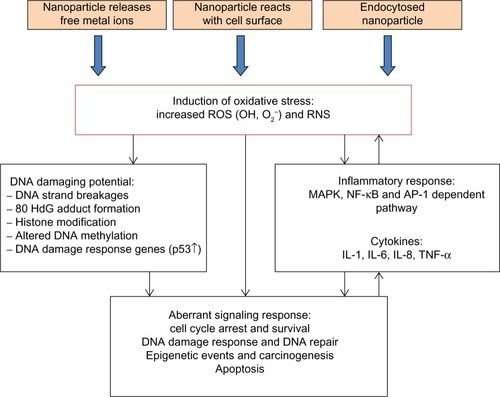
Figure 1 Various states of nanoparticles in different forms of dry powder and liquid in suspension media.
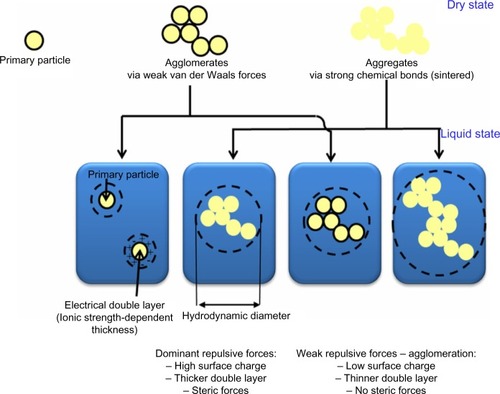
Table 1 Genotoxicity studies of ZnO and silica nanoparaticles using in vitro and in vivo mammalian models
Figure 2 Scheme illustrating possible routes of cellular uptake, including passive diffusion, receptor-related endocytosis, and clarthrin- or caveolae-dependent endocytosis. In brief, nanoparticles are in the correct size and shape. They may dock on membrane receptors, facilitating receptor-mediated endocytosis. Alternatively, clathrin- or caveolae-mediated endocytosis may occur, which results in the formation of pits in the region of 120 nm or up to 80 nm, respectively, which regulates the size of the material they are able to enclose.
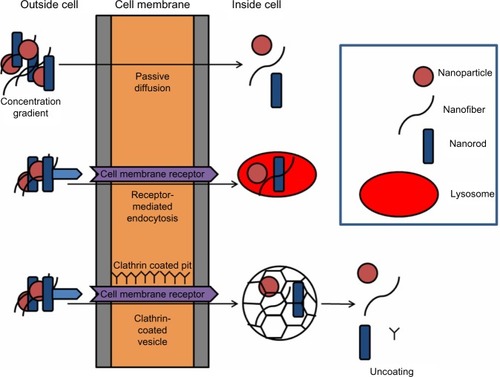
Figure 3 Key indirect mechanisms underlying nanogenotoxicity. Nanoparticles (NPs) may cause oxidative stress induction, inflammatory responses, or aberrant cellular signaling. These responses may be implicated in cancer risk.
Abbreviations: NPs, nanoparticles; ROS, reactive oxygen species.
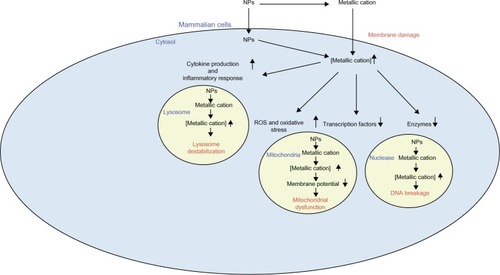
Figure 4 Putative mechanisms underlying the detrimental effects of zinc oxide and silica nanoparticles. These nanoparticles dissolve in the extracellular milieu, giving rise to increased extracellular metallic cations. This leads to increased intracellular respective metallic cations, resulting in decreased activity of particular enzymes and transcription factors. Moreover, this event can induce ROS generation and resulting oxidative stress, as well as stimulate various cytokine production and inflammatory responses. These phenomena, in turn, render membrane damage, DNA breakage, mitochondrial dysfunction, and lysosome destabilization.
Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species; IL, interleukin; TNF, tumor necrosis factor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, mitogen-activated protein kinases; AP-1, activator protein 1.