Figures & data
Table 1 Properties of multi-walled carbon nanotubes (MWCNTs)
Table 2 Properties of cup-stacked carbon nanotubes (CSCNTs)
Figure 1 Cell viability upon exposure to CNTs.
Notes: Cells were exposed to the indicated concentrations of CNT for 24 hours. BEAS-2B cells exposed to (A) MWCNTs (VGCF®-X, VGCF®-S, and VGCF®) and (B) CSCNTs (CS-L, CS-S, and CS-M). MESO-1 cells exposed to (C) MWCNTs and (D) CSCNTs. DM (0.001% gelatin) served as the control, and data are expressed as mean ± standard error (n=6). *P<0.05; **P<0.01; ***P<0.001. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan); VGCF, vapor grown carbon fibers; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length.
Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked CNT; DM, dispersant medium; MWCNT, multi-walled CNT; VGCF, vapor grown carbon fibers; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length.
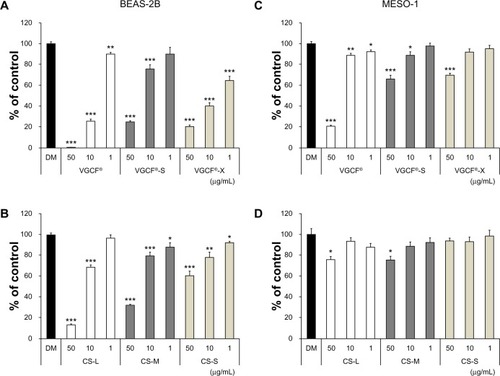
Figure 2 Plasma membrane permeability in cells exposed to CNTs.
Notes: Cells were exposed to 10 μg/mL CNT for 24 hours. (A) BEAS-2B and (B) MESO-1 cells exposed to MWCNTs or CSCNTs. The LDH activity was calculated by the formula ([experimental value − DM value]/[PC value − DM value]) ×10 × 100 (%). PC is 0.01% Triton X-100; DM is 0.001% gelatin. Data are compared to the control (DM) and expressed as mean ± standard error (n=3). *P<0.05; **P<0.01; ***P<0.001. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).
Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked CNT; DM, dispersant medium; LDH, lactate dehydrogenase; MWCNT, multi-walled CNT; PC, positive control; VGCF, vapor grown carbon fibers; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length.
![Figure 2 Plasma membrane permeability in cells exposed to CNTs.Notes: Cells were exposed to 10 μg/mL CNT for 24 hours. (A) BEAS-2B and (B) MESO-1 cells exposed to MWCNTs or CSCNTs. The LDH activity was calculated by the formula ([experimental value − DM value]/[PC value − DM value]) ×10 × 100 (%). PC is 0.01% Triton X-100; DM is 0.001% gelatin. Data are compared to the control (DM) and expressed as mean ± standard error (n=3). *P<0.05; **P<0.01; ***P<0.001. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked CNT; DM, dispersant medium; LDH, lactate dehydrogenase; MWCNT, multi-walled CNT; PC, positive control; VGCF, vapor grown carbon fibers; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length.](/cms/asset/90665ce3-cc3f-4f15-bf11-5fd6c5678577/dijn_a_58661_f0002_c.jpg)
Figure 3 Live cells imaged by differential interference contrast optics after incubation with CellLight® Lysosomes-RFP and Early Endosomes-GFP (Life Technologies, Carlsbad, CA, USA) and bisbenzimide H33342 fluorochrome trihydrochloride for nuclear staining.
Notes: (A) BEAS-2B cells were exposed to 1 μg/mL MWCNT (VGCF) or CSCNT (CS) for 24 hours. (a) DM (control); (b) VGCF®; (c) VGCF®-S; (d) VGCF®-X; (e) CS-L; (f) CS-M; and (g) CS-S. Red arrow indicates VGCF-X agglomerates, which were not taken up by BEAS-2B cells, nor did they fully penetrate the cell membrane. Scale bar =10 μm. (B) MESO-1 cells were exposed to 10 μg/mL MWCNT (VGCF) or CSCNT (CS) for 24 hours. (a) DM (control); (b) VGCF; (c) VGCF-S; (d) VGCF-X; (e) CS-L; (f) CS-M; and (g) CS-S. Scale bar =10 μm. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).
Abbreviations: CSCNT, cup-stacked carbon nanotube; DM, dispersant medium; MWCNT, multi-walled carbon nanotube; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length.
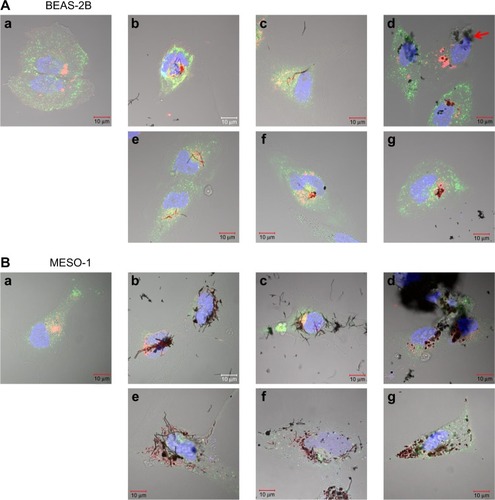
Figure 4 Transmission electron micrographs of BEAS-2B and MESO-1 cells exposed to CNTs.
Notes: (A) BEAS-2B cells were exposed to 1 μg/mL MWCNT (VGCF) or CSCNT (CS) for 24 hours. (a) DM (control); (b and c) VGCF®; (d and e) VGCF®-S; (f and g) VGCF®-X; (h and i) CS-L; (j and k) CS-M; and (l and m) CS-S. Red arrow indicates VGCF-X agglomerates, which were not taken up by BEAS-2B cells, nor did they fully penetrate the cell membrane. (B) MESO-1 cells were exposed to 10 μg/mL MWCNT or CSCNT for 24 hours. (a) DM (control); (b and c) VGCF; (d and e) VGCF-S; (f and g) VGCF-X; (h and i) CS-L; (j and k) CS-M; and (l and m) CS-S. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).
Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked carbon nanotube; DM, dispersant medium; MWCNT, multi-walled carbon nanotube; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length; VGCF, vapor grown carbon fibers.
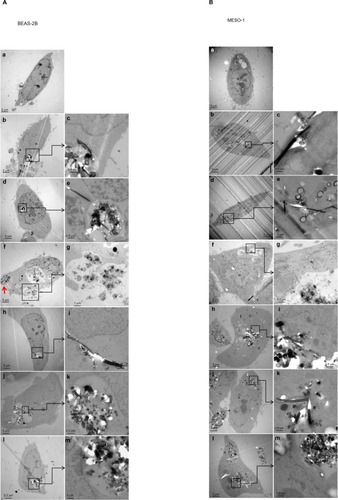
Figure 5 Total reactive oxygen species production upon exposure to CNTs.
Notes: (A) BEAS-2B cells were exposed to 1 μg/mL MWCNT (VGCF®, VGCF®-S, and VGCF®-X) or CSCNT (CS-L, CS-M, and CS-S) for 1 hour. (B) MESO-1 cells were exposed to 10 μg/mL MWCNT (VGCF, VGCF-S, and VGCF-X) or CSCNT (CS-L, CS-M, and CS-S) for 1 hour. Pyocyanin (100 μM) was used to induce the production of reactive oxygen species. Data are expressed as mean ± standard error (n=3). *P<0.05; **P<0.01; ***P<0.001. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).
Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked carbon nanotube; DM, dispersant medium; MWCNT, multi-walled carbon nanotube; OSDR, oxidative stress detection reagent; SDR, superoxide detection reagent; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length; VGCF, vapor grown carbon fibers.
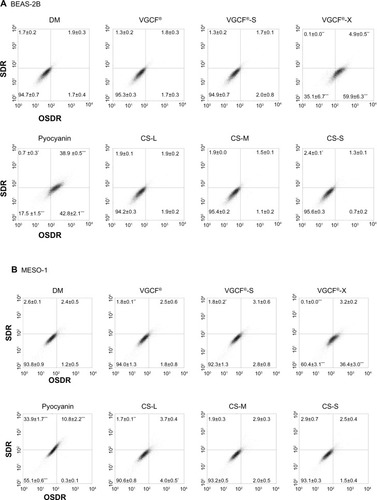
Figure 6 Intracellular acidification upon exposure to CNTs.
Notes: (A) BEAS-2B cells were exposed to 1 and 10 μg/mL of MWCNT (VGCF®-X, VGCF®-S, and VGCF®) or CSCNT (CS-L, CS-S, and CS-M) for 24 hours. (B) MESO-1 cells were exposed to 1, 10, and 50 μg/mL MWCNT or CSCNT for 24 hours, incubated with an acidotropic probe (LysoSensor™; Life Technologies, Carlsbad, CA, USA), and analyzed by flow cytometry (10,000 cells). The LysoSensor intensity (%) was calculated as follows: ([FL1 channel intensity of cells exposed to CNTs − FL1 channel intensity of untreated cells]/[FL1 channel intensity of cells exposed to DM − FL1 channel intensity of untreated cells]) ×100%. DM is 0.001% gelatin. CNT inhibition intensity (%) was calculated as follows: ([FL1 channel intensity of CNT blank − FL1 channel intensity of untreated cells]/[FL1 channel intensity of DM blank − FL1 channel intensity of untreated cells]) ×100%. The intensity change was determined as follows: intensity (Δ%) = LysoSensor intensity (%) − CNT inhibition intensity (%). Data are expressed as mean ± standard error (n=4). (C) BEAS-2B cells were exposed to 10 μg/mL MWCNT (VGCF, VGCF-S, and VGCF-X) or CSCNT (CS-L, CS-M, and CS-S) for 24 hours and visualized by staining with LysoSensor dye (green), CellLight® Lysosomes-RFP (red [Life Technologies]), and bisbenzimide H33342 fluorochrome trihydrochloride (blue [Nacalai Tesque, Kyoto, Japan]) for nuclei. Scale bar =20 μm. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).
Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked carbon nanotube; DM, dispersant medium; MWCNT, multi-walled carbon nanotube; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length; VGCF, vapor grown carbon fibers.
![Figure 6 Intracellular acidification upon exposure to CNTs.Notes: (A) BEAS-2B cells were exposed to 1 and 10 μg/mL of MWCNT (VGCF®-X, VGCF®-S, and VGCF®) or CSCNT (CS-L, CS-S, and CS-M) for 24 hours. (B) MESO-1 cells were exposed to 1, 10, and 50 μg/mL MWCNT or CSCNT for 24 hours, incubated with an acidotropic probe (LysoSensor™; Life Technologies, Carlsbad, CA, USA), and analyzed by flow cytometry (10,000 cells). The LysoSensor intensity (%) was calculated as follows: ([FL1 channel intensity of cells exposed to CNTs − FL1 channel intensity of untreated cells]/[FL1 channel intensity of cells exposed to DM − FL1 channel intensity of untreated cells]) ×100%. DM is 0.001% gelatin. CNT inhibition intensity (%) was calculated as follows: ([FL1 channel intensity of CNT blank − FL1 channel intensity of untreated cells]/[FL1 channel intensity of DM blank − FL1 channel intensity of untreated cells]) ×100%. The intensity change was determined as follows: intensity (Δ%) = LysoSensor intensity (%) − CNT inhibition intensity (%). Data are expressed as mean ± standard error (n=4). (C) BEAS-2B cells were exposed to 10 μg/mL MWCNT (VGCF, VGCF-S, and VGCF-X) or CSCNT (CS-L, CS-M, and CS-S) for 24 hours and visualized by staining with LysoSensor dye (green), CellLight® Lysosomes-RFP (red [Life Technologies]), and bisbenzimide H33342 fluorochrome trihydrochloride (blue [Nacalai Tesque, Kyoto, Japan]) for nuclei. Scale bar =20 μm. MWCNTs were provided by Showa Denko KK (Tokyo, Japan); CSCNTs were provided by GSI Creos (Tokyo, Japan).Abbreviations: CNT, carbon nanotube; CSCNT, cup-stacked carbon nanotube; DM, dispersant medium; MWCNT, multi-walled carbon nanotube; CS-L, CSCNT of length 20–80 μm; CS-S, CSCNT of length 0.5–20 μm; CS-M, CSCNT of intermediate length; VGCF, vapor grown carbon fibers.](/cms/asset/bcf36fc7-0ac7-4872-9b98-367b8202f015/dijn_a_58661_f0006_c.jpg)
