Figures & data
Table 1 The compositions of electrospining solutions
Figure 1 The morphology and elemental composition of the four scaffolds.
Notes: Scanning electron micrographs and their EDX spectra of the four fibrous scaffolds. (A) COL/PCL with interconnected pores and smooth surface; (B) COL/PCL/nHA with a rough surface and a diameter of about 377 nm; (C) COL/PCL-SBF, few CaP deposits could be seen on the fiber surface; (D) COL/PCL/nHA-SBF, CaP precipitation was visible. The results of the EDX spectroscopy analysis was also consistent with SEM.
Abbreviations: SEM, scanning electron microscopy; EDX, energy dispersive X-ray spectroscopy; COL/PCL, the electrospun type-I collagen/poly(ε-caprolactone) scaffold; COL/PCL/nHA, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold; COL/PCL-SBF, the electrospun type-I collagen/poly(ε-caprolactone) scaffold immersed in simulated body fluid; COL/PCL/nHA-SBF, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold immersed in simulated body fluid; CaP, calcium and phosphorus compound.
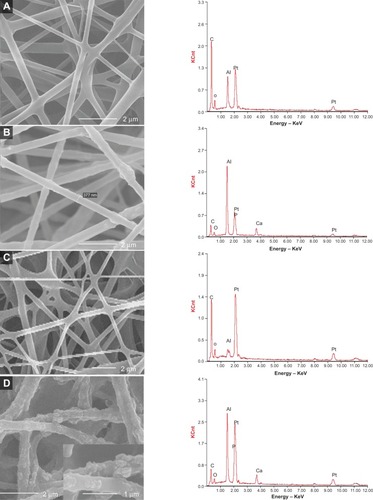
Figure 2 Water contact angles of COL/PCL-SBF, COL/PCL/nHA-SBF and their data presented as mean values ± standard deviation.
Notes: The water contact angle of COL/PCL-SBF is about 117.35° (A). The water contact angle of COL/PCL/nHA-SBF is about 67.43° (B). Additionally, their statistical data presented as mean ± standard deviation; *P<0.05 (COL/PCL-SBF versus COL/PCL/nHA-SBF) (C).
Abbreviations: COL/PCL-SBF, the electrospun type-I collagen/poly(ε-caprolactone) scaffold immersed in simulated body fluid; COL/PCL/nHA-SBF, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold immersed in simulated body fluid.
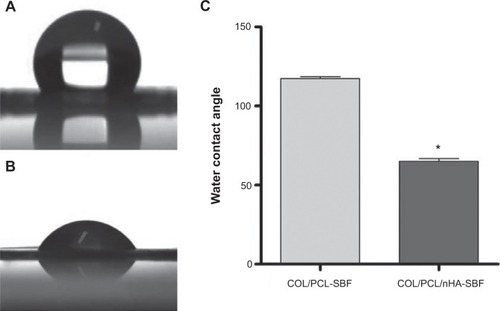
Figure 3 Scanning electron micrographs of PDLCs cultured on the COL/PCL-SBF and COL/PCL/nHA-SBF scaffolds.
Notes: Cells on the COL/PCL-SBF (A, B, C) and COL/PCL/nHA-SBF (D, E, F) appeared to have no significant difference in morphology. The cells adhered to the fibers were spindle-shaped on the first day (A, D), and then extended gradually and adequately at day 3 (B, E). At day 8, the PDLCs were further flattened and stretched out flopodial (C, F).
Abbreviations: COL/PCL-SBF, the electrospun type-I collagen/poly(ε-caprolactone) scaffold immersed in simulated body fluid; COL/PCL/nHA-SBF, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold immersed in simulated body fluid.
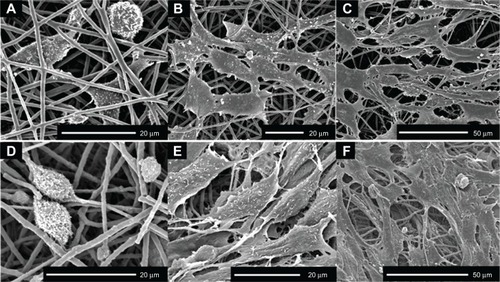
Figure 4 PDLC proliferation on the COL/PCL-SBF and COL/PCL/nHA-SBF scaffolds.
Notes: Data represent mean ± standard deviation. No significant difference against cell proliferation is shown on the two scaffolds (COL/PCL-SBF versus COL/PCL/nHA-SBF).
Abbreviations: COL/PCL-SBF, the electrospun type-I collagen/poly(ε-caprolactone) scaffold immersed in simulated body fluid; COL/PCL/nHA-SBF, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold immersed in simulated body fluid.
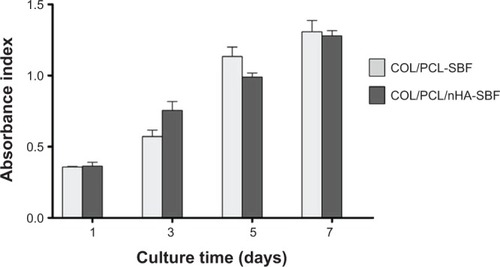
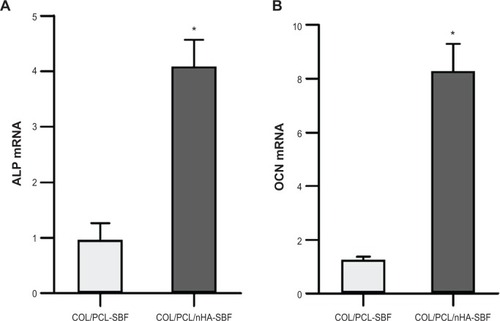
Figure 5 Real-time polymerase chain reaction analysis of ALP and OCN messenger ribonucleic acid expression in periodontal ligament cells on two scaffolds.
Notes: Gene expression was normalized to housekeeping gene β-actin expression. Periodontal ligament cells were cultivated for 10 days on the COL/PCL-SBF and COL/PCL/nHA-SBF scffolds. Data represents mean ± standard deviation (n=3); *P<0.05 (COL/PCL-SBF versus COL/PCL/nHA-SBF).
Abbreviations: ALP, phosphatase; OCN, osteocalcin, COL/PCL-SBF, the electrospun type-I collagen/poly(ε-caprolactone) scaffold immersed in simulated body fluid; COL/PCL/nHA-SBF, the electrospun type-I collagen/poly(ε-caprolactone)/nanoscale hydroxyapatite scaffold immersed in simulated body fluid.