Figures & data
Figure 1 Preparation and characterization of magnetoliposomes containing recombinant human IFNα2b (MIL).
Notes: (A) Mean diameters of MIL were determined using dynamic light scattering with different molar ratios of the following lipids: phosphatidylcholine (PC), cholesterol (Chol), and 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG), in Tris buffer at 25°C. (B) Plot of polydispersity indexes of MIL under different molar ratios of PC/Chol/DMPG. (C) Plot of drug-encapsulation efficiency of MIL under different molar ratios of PC/Chol/DMPG. (D) Plot of MIL yields with different molar ratios of PC/Chol/DMPG. (E) Transmission electron microscopy image of Fe3O4. Bar =50 nm; the mean diameter of Fe3O4 was 10 nm. (F) Transmission electron microscopy image of MIL. Bar =200 nm; the mean diameter of MIL was 170 nm. *Significant difference when compared with lipid composition 6:3:0 (P<0.05).
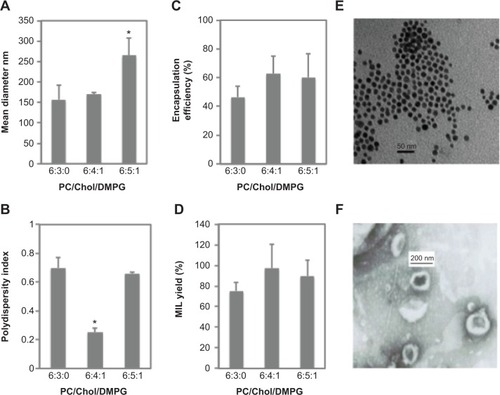
Table 1 Magnetoliposomes containing recombinant human IFNα2b hemolysis test results (spectrophotometry) (n=5)
Figure 2 Pharmacokinetic and tissue-distribution studies of magnetoliposomes containing recombinant human (rh)-IFNα2b (MIL) in animals.
Notes: (A) Plot of plasma rhIFNα2b concentrations at eight different time points (10, 30, 60, 120, 180, 240, 300, and 480 minutes) from three groups of mice intravenously injected with free rhIFNα2b (group A), and MIL (groups B and C). Group C mice were given external magnetic tablets, whereas group B mice were not. (B) Plot of rhIFNα2b concentrations in spleen tissues. (C) Plot of rhIFNα2b concentrations in liver tissues. (D) Plot of rhIFNα2b concentrations in kidney tissues. (E) Plot of rhIFNα2b concentrations in lung tissues. (F) Plot of rhIFNα2b concentrations in heart tissues.
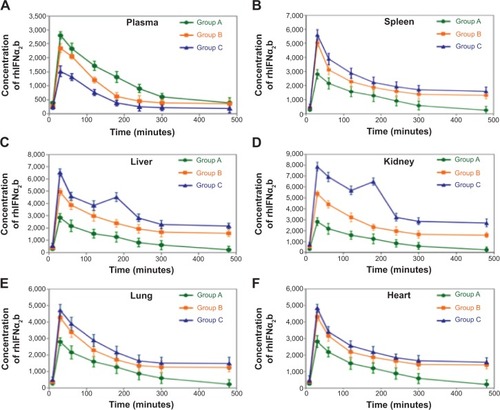
Figure 3 Magnetoliposomes (MLs) containing recombinant human (rh)-IFNα2b (MIL) inhibit the growth of hepatoma cancer Bel-7402 cells.
Notes: (A) Inhibitory rates of Bel-7402 cells in the experimental (ML, IFNα2b [IFN], IFNα2b liposome [IL], and MIL) groups and control (NS) group. (B) Acridine orange test results of Bel-7402 cells treated with NS, IFN, IL, ML, and MIL for 24 hours. (C) Concentrations of p-nitroanilide (pNA) from five different groups treated with NS, IFN, IL, ML, and MIL were determined by measuring optical density values at 405 nm with colorimetric kits. *Significant difference when compared with the NS group (P<0.05).
![Figure 3 Magnetoliposomes (MLs) containing recombinant human (rh)-IFNα2b (MIL) inhibit the growth of hepatoma cancer Bel-7402 cells.Notes: (A) Inhibitory rates of Bel-7402 cells in the experimental (ML, IFNα2b [IFN], IFNα2b liposome [IL], and MIL) groups and control (NS) group. (B) Acridine orange test results of Bel-7402 cells treated with NS, IFN, IL, ML, and MIL for 24 hours. (C) Concentrations of p-nitroanilide (pNA) from five different groups treated with NS, IFN, IL, ML, and MIL were determined by measuring optical density values at 405 nm with colorimetric kits. *Significant difference when compared with the NS group (P<0.05).](/cms/asset/7ea02ee0-cd09-4b76-9c17-f04c3dc64e47/dijn_a_67228_f0003_c.jpg)
Figure 4 Magnetoliposomes (MLs) containing recombinant human (rh)-IFNα2b (MIL) inhibit liver tumor growth.
Notes: (A) Tumors collected from nude mice in different groups (n=6). (B) Plot of tumor masses from nude mice in different groups. (C) Comparison of tumor volumes of nude mice among different groups (n=6): saline NS, containing 0 IU rh-IFNα2b; ML, containing 0 IU rh-IFNα2b; IFN, containing 10,000 IU rh-IFNα2b; IL, containing 1,000 IU rh-IFNα2b; and MIL, containing 1,000 IU rh-IFNα2b. *Significant difference when compared with the NS group (P<0.05).
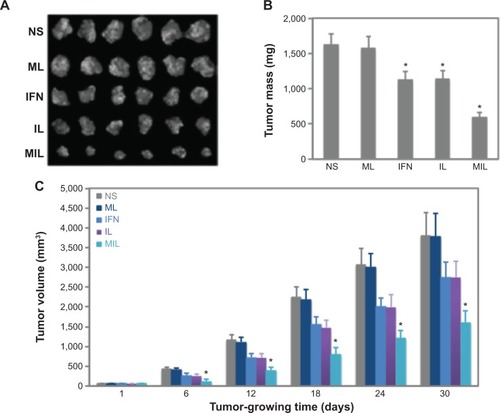
Figure 5 Magnetoliposomes (MLs) containing recombinant human IFNα2b (MIL) activate cell apoptosis in liver tumors.
Notes: (A) Quantitative real-time reverse-transcription polymerase chain-reaction results show the transcription levels of Bax and Bcl-2 in tumor samples obtained from mice of five different groups (saline [NS], IFNα2b [IFN], IFNα2b liposome [IL], ML, and MIL groups). The right panel shows the relative messenger ribonucleic acid (mRNA) levels of Bax in the five groups, whereas the left panel shows the relative mRNA levels of Bcl-2 in the five groups. (B) Ratio of Bax/Bcl-2-transcription levels calculated from results of (A) in each group. (C) Western blot analysis shows Bax- and Bcl-2-expression levels in tumor tissues obtained from nude mice in different groups (NS, IFN, IL, ML, and MIL). (D) Ratio of Bax/Bcl-2 protein levels in tumor tissues of nude mice among the different groups, as determined from results of (C). *Significant difference when compared with the NS group (P<0.05).
![Figure 5 Magnetoliposomes (MLs) containing recombinant human IFNα2b (MIL) activate cell apoptosis in liver tumors.Notes: (A) Quantitative real-time reverse-transcription polymerase chain-reaction results show the transcription levels of Bax and Bcl-2 in tumor samples obtained from mice of five different groups (saline [NS], IFNα2b [IFN], IFNα2b liposome [IL], ML, and MIL groups). The right panel shows the relative messenger ribonucleic acid (mRNA) levels of Bax in the five groups, whereas the left panel shows the relative mRNA levels of Bcl-2 in the five groups. (B) Ratio of Bax/Bcl-2-transcription levels calculated from results of (A) in each group. (C) Western blot analysis shows Bax- and Bcl-2-expression levels in tumor tissues obtained from nude mice in different groups (NS, IFN, IL, ML, and MIL). (D) Ratio of Bax/Bcl-2 protein levels in tumor tissues of nude mice among the different groups, as determined from results of (C). *Significant difference when compared with the NS group (P<0.05).](/cms/asset/31d9dc90-eecf-47ee-bcda-af20df34f2e1/dijn_a_67228_f0005_c.jpg)
Figure S1 The elution profile shows that magnetoliposomes containing recombinant human (rh)-IFNα2b (MIL) were purified from the mixture using Sephadex G-50 column chromatography. Two peaks including MIL particles and free IFNα2b are shown here.
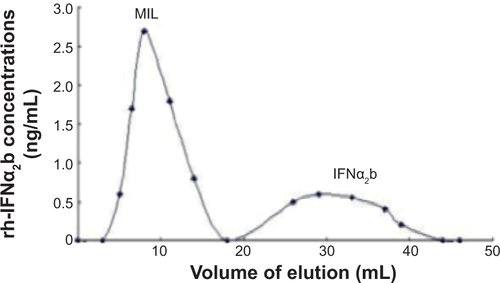
Figure S2 Mean diameters of magnetoliposomes containing recombinant human IFNα2b (MIL) were determined using dynamic light scattering. As shown, the mean diameter of MIL was approximately 170 nm.
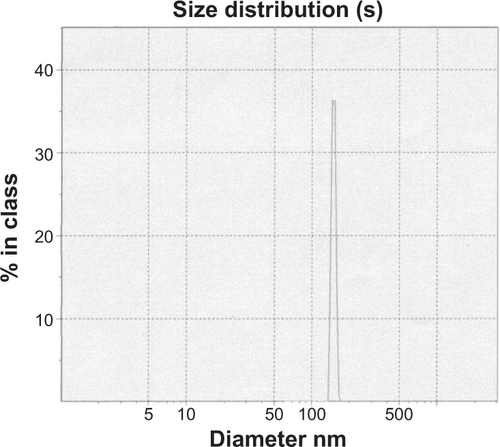
Figure S3 The behavior of magnetoliposomes containing recombinant human IFNα2b (MIL) in solution with or without an external magnetic field.
Notes: (A) MIL was accumulated at one side of the bottle under the external magnetic field. (B) MIL was distributed in the solution when no magnet tablet was provided.
Figure S4 Magnetoliposomes containing recombinant human IFNα2b (MIL) were stable enough when stored for a long time or at low temperature.
Notes: (A) The mean diameter of MIL did not show significant difference when MIL was stored for 6 months at −20°C. (B) The drug-entrapment efficiency did not change significantly when MIL was stored for 6 months at −20°C.
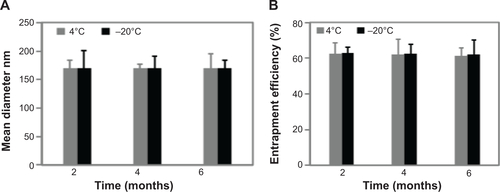
Figure S5 Effect of irradiation sterilization on magnetoliposomes containing recombinant human IFNα2b (MIL).
Notes: (A) The mean diameter of MIL particles before and after irradiation sterilization. P1 represents MIL particles before irradiation sterilization, while P2 is the MIL particles after irradiation sterilization. (B) Effect of irradiation sterilization on the drug-entrapment efficiency of MIL. E1 is the entrapment efficiency before sterilization, while E2 represents the entrapment efficiency after irradiation sterilization.
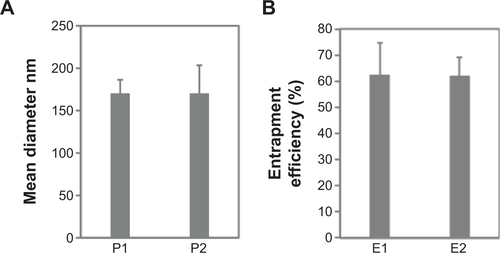
Figure S6 Effects of magnetoliposomes containing recombinant human (rh)-IFNα2b (MIL) on platelets and leukocytes in blood.
Notes: (A) Platelet-aggregation rates from seven different groups were shown. Here, group 1 is the control group (NS), in which platelets were treated with saline, whereas groups 2–7 are the platelets treated with MIL containing the following concentrations of rhIFNα2b: 2,000, 4,000, 6,000, 8,000, 10,000, and 12,000 IU/mL. (B) Numbers of white blood cells (WBCs) from seven different groups are plotted. The seven groups are as described in A. (C) Polymorphonuclear leucocytes (PMNs) from the same seven groups are plotted. (D) Lymphocytes (LYMs) from the same seven groups are also plotted. (E) Nitroblue tetrazolium (NBT)-positive cells were determined using an NBT-reducing test. The results reflect the phagocytic activities of leukocytes in different groups.
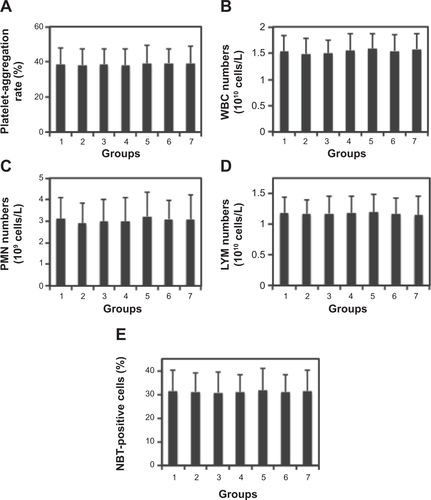
Table S1 Observation of animal acute toxicity
Table S2 Body weights of mice in acute-toxicity test
