Figures & data
Figure 1 Schematic illustration of culture models used in this study.
Notes: (A) To evaluate the bioactivity (neurite outgrowth inductive potential) of np-NTFs, half-cut adult DRG explants were maintained for 3 days in SFM at 37°C with 5% CO2 and sister cultures were stimulated with three different quantities (10 ng/mL, 50 ng/mL, or 100 ng/mL) of each single np-NTF (np-NGF, np-FGF-218kDa, or np-GDNF) or with a pool of the three np-NTFs (final concentration of each np-NTF 30 ng/mL or 50 ng/mL). (B, upper row) Neonatal DRGs were dissociated and seeded as a drop (2,500 neurons in 5 μL medium) into a layer of 800 or 1,000 μL of NVR-Gel (pure or supplemented with either single free recombinant NTFs or np-NTFs or a pool of either the free NTFs or np-NTFs). (B, lower row) In addition, DRG drop cultures were seeded into NVR-Gel containing nontransfected BMSCs or BMSCs overexpressing selected NTFs at a density of 40×104 cells/1,000 μL of 0.5% NVR-Gel. Cultures were maintained for 1 day. (C) A density of 30×104 EGFP-PC-12 cells was mixed with either np-NTFs or 40×104 nT-BMSCs or FGF-218kDa-BMSCs in a volume of 1 mL 0.5% NVR-Gel (containing PC-12 cell differentiation medium) and transferred into a 24-multiwell culture well. Cultures were maintained for 5 days.
Abbreviations: np, nanoparticle; NTFs, neurotrophic factors; DRG, dorsal root ganglion; SFM, serum-free medium; NGF, nerve growth factor; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor; BMSCs, bone marrow-derived mesenchymal stromal cells; EGFP, enhanced green fluorescent protein; PC-12 cells, cell line from rat pheochromocytoma cells; PSN, penicillin–streptomycin–neomycin; BSA, bovine serum albumin; DMEM, Dulbecco’s Modified Eagle’s Medium; MEM, Minimum Essential Medium; FCS, fetal calf serum; pen/strep, penicillin/streptomycin.
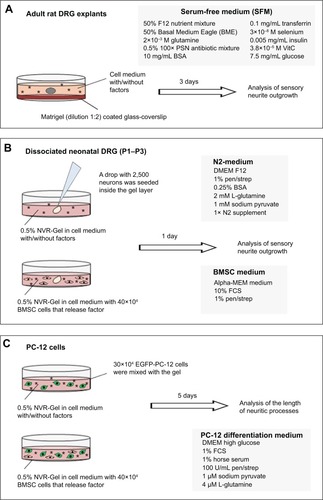
Figure 2 Conjugation of NTFs to iron oxide nanoparticles maintains their bioactivity over 2 weeks in vitro.
Notes: (A) Bar graph depicting the number of neuritic extensions from adult DRG explants (at distance 0 μm) treated with free GDNF or np-GDNF. Medium formulations were used either freshly prepared or after a period of in vitro preincubation of 2 weeks. Fresh free GDNF has the strongest effect on numeric neurite outgrowth. However, during in vitro preincubation, free GDNF loses all its bioactivity, while np-GDNF maintains its increased neurite inductivity in comparison with control conditions. Bars represent mean values ± standard deviations (*P≤0.05, **P≤0.01). (B) Line graph representing the number of intersections of the neuritic processes with circles drawn in distinct distances from the center of the DRGs (x-axis) and their distance from the center of the DRG explants (y-axis). (C) Representative photomicrographs of adult DRG explant cultures in SFM supplemented with 50 ng/mL of free GDNF or np-GDNF freshly prepared or after a period of in vitro preincubation of 2 weeks. Neurites marked with anti-βIII-tubulin in green, scale bars: 200 μm.
Abbreviations: NTFs, neurotrophic factors; DRG, dorsal root ganglion; GDNF, glia-derived neurotrophic factor; np, nanoparticle; SFM, serum-free medium; CTR, control.
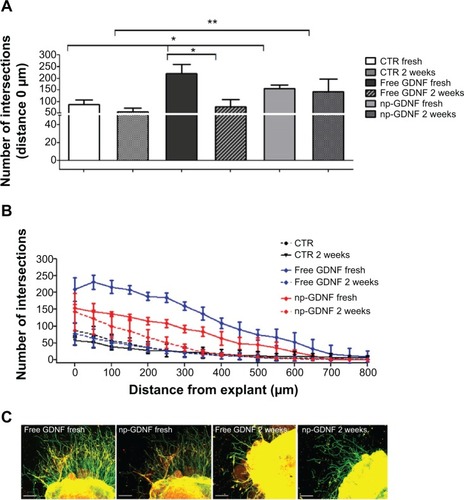
Figure 3 After incorporation into NVR-Gel, np-NTFs demonstrate the same bioactivity as free NTFs.
Notes: (Left column) Representative photomicrographs of neonatal DRG drop cultures in NVR-Gel supplemented for 24 hours with 50 ng/mL of np-NGF (A), np-FGF-218kDa (C), or np-GDNF (E). Neurites marked with anti-βIII-tubulin in red, Schwann cells marked with anti-S100 in green, and nuclear staining (DAPI) in blue. Scale bars: 500 μm. (Right column) Bar graphs depicting the number of neurite intersections (y-axis) with circles drawn at distinct distances (x-axis) from the neonatal DRG drop culture. The neurite inductive effect of each free NTF was compared with its np-NTF counterpart and the nontreated CTR: (B) NGF, (D) FGF-218kDa, (F) GDNF (*P≤0.05, **P≤0.01, ***P≤0.001). (G) Bar graph illustrating the mean length ± standard deviation of the ten longest neurites extending from each DRG drop culture. No significant difference was detectable among all treatments.
Abbreviations: np, nanoparticle; NTFs, neurotrophic factors; DRG, dorsal root ganglion; NGF, nerve growth factor; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; CTR, control.
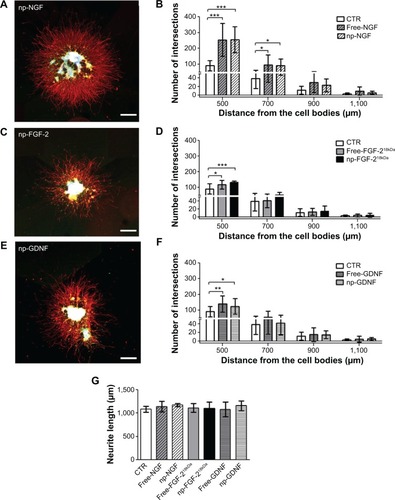
Figure 4 Western blot results for protein detection after nonviral transfection of BMSCs.
Notes: (A) Analysis of cell lysates of nT-BMSCs or genetically engineered empty-BMSCs, FGF-218kDa-BMSCs, or GDNF-BMSCs (24 hours, α-FGF-2, and α-GDNF antibody). (B) Analysis of cell culture supernatants from nT-BMSCs or genetically engineered empty-BMSCs, or GDNF-BMSCs harvested after 24 hours, 72 hours, and 120 hours (α-GDNF antibody).
Abbreviations: BMSCs, bone marrow-derived mesenchymal stromal cells; np, nanoparticle; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor; nT, nontransfected.
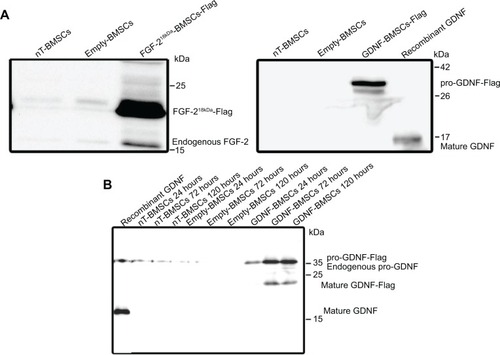
Figure 5 Engineered neurotrophic factor-overexpressing BMSCs have less neurite inductive bioactivity in DRG cultures than nontransfected CTR cells.
Notes: Neurite outgrowth was quantitatively analyzed with regard to (A) the number of neurite intersections (y-axis) with circles drawn at distinct distances (x-axis) from the neonatal DRG drop culture co-cultured with nT-BMSCs, FGF-218kDa-BMSCs, or GDNF-BMSCs and (B) mean length ± standard deviation of the ten longest neurites extending from each DRG drop culture. (C) Sample photomicrograph of fluorescence-labeled DRG drop culture (β-III-tubulin, green) co-cultured with nT-BMSCs (F-actin-phalloidin, red).
Abbreviations: BMSCs, bone marrow-derived mesenchymal stromal cells; DRG, dorsal root ganglion; CTR, control; nT, nontransfected; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor.
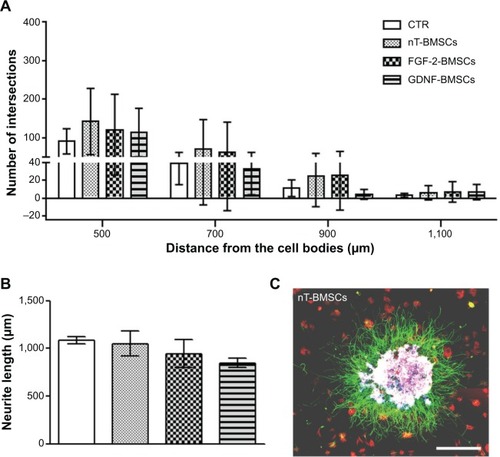
Figure 6 Engineered neurotrophic factor-overexpressing BMSCs have less neurite inductive bioactivity than np-NTFs.
Notes: (A) Bar graphs depicting the number of neurite intersections from the neonatal DRG drop culture under CTR conditions or treatment with 50 ng/mL np-NTFs or co-cultured with genetically engineered BMSCs. (B) Bar graph illustrating the mean length ± standard deviation of the ten longest neurites extending from each neonatal DRG drop culture.
Abbreviations: BMSCs, bone marrow-derived mesenchymal stromal cells; np, nanoparticle; NTFs, neurotrophic factors; DRG, dorsal root ganglion; CTR, control; nT, nontransfected; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor.
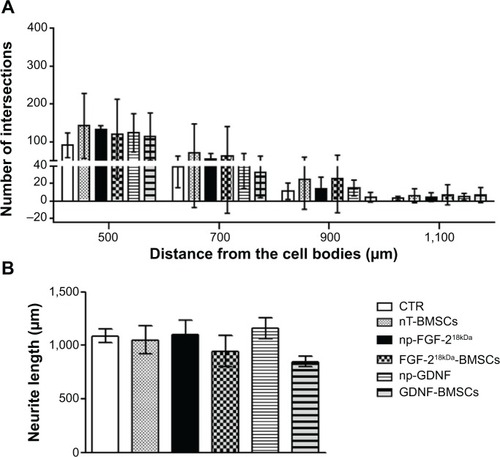
Figure 7 Neuronal differentiation of PC-12 cells is seen to the highest extent in co-cultures with FGF-218kDa-BMSCs.
Notes: (A) Neurite formation by EGFP-PC-12 cells was quantitatively analyzed in the presence of either 50 ng/mL of free NTFs (NGF or FGF-218kDa) or np-NTFs (NGF or FGF-218kDa) or in co-culture of FGF-218kDa-BMSCs. While no significant differences were detectable between treatment with free NTFs or np-NTFs, nT-BMSCs demonstrated a significantly reduced neurite inductive bioactivity in comparison with all other conditions (**P≤0.01, ***P≤0.001). In contrast, maximal neuronal differentiation by PC-12 cells was detected in co-culture with FGF-218kDa-BMSCs, with significant difference to free FGF-218kDa and np-FGF-218kDa (#P≤0.05). (B) Sample photomicrograph of EGFP-PC-12 cells co-cultured with FGF-218kDa-BMSCs.
Abbreviations: PC-12 cells, cell line from rat pheochromocytoma cells; FGF, fibroblast growth factor; BMSCs, bone marrow-derived mesenchymal stromal cells; EGFP, enhanced green fluorescent protein; NTFs, neurotrophic factors; NGF, nerve growth factor; np, nanoparticle; nT, nontransfected.
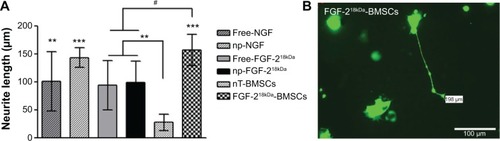
Figure S1 Nontransfected and EGFP-Flag-BMSCs in culture.
Notes: (A) nT-BMSCs stained for F-actin filaments (red), demonstrating their regular fibroblast-like shape. (B) Anti-Flag (red) stained BMSCs after nonviral transfection with EGFP-Flag. Nuclear staining with 4′,6-diamidino-2-phenylindole (blue).
Abbreviations: EGFP, enhanced green fluorescent protein; BMSCs, bone marrow-derived mesenchymal stromal cells; nT, nontransfected.
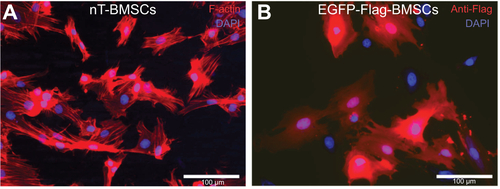
Figure S2 Neurotrophic factors conjugated to iron oxide nanoparticles stimulate neurite outgrowth from adult DRG explants in a dose-dependent manner.
Notes: (A) Bar graph representing the number of neuritic extensions from adult DRG explants (at distance 0 μm) treated with different amounts of np-NTFs. Statistical analysis revealed the strongest effect on neurite outgrowth when explants were treated with 50 ng/mL of either np-NGF or np-GDNF. Treatment with 10 ng/mL np-FGF-218kDa already evoked a significant increase of neurite outgrowth when compared with untreated controls. Bars represent mean values ± standard deviations (**P≤0.01, ***P≤0.001). (B) Line graph representing the number of intersections of the neuritic processes with circles drawn in distinct distances from the center of the DRGs (x-axis) and their distance from the center of the DRG explants (y-axis).
Abbreviations: DRG, dorsal root ganglion; np, nanoparticle; NTFs, neurotrophic factors; NGF, nerve growth factor; GDNF, glia-derived neurotrophic factor; FGF, fibroblast growth factor; CTR, control.
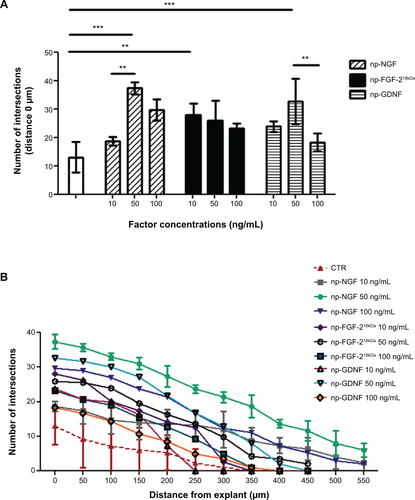
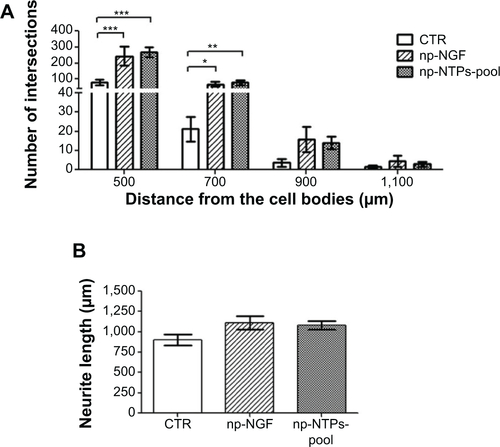
Figure S3 Combined administration of the three np-NTFs fails to increase the number of neurites extending from adult DRG explants.
Notes: (A) Bar graph representing the number of neuritic extensions of adult DRG explants (at distance 0 μm) treated with np-NGF (50 ng/mL) or with an np-NTF pool (np-NGF + np-FGF-218kDa + np-GDNF) in the concentration of 30 ng/mL (each np-NTF) or 50 ng/mL (each np-NTF), respectively. Bars represent mean values ± standard deviations (*P≤0.05, ***P≤0.001). (B) Line graph representing the number of intersections of the neuritic processes with circles drawn in distinct distances from the center of the DRGs (x-axis) and their distance from the center of the DRG explants (y-axis). No significant increase over the neurite outgrowth inductive effect seen with treatment with 50 ng/mL np-NGF alone could be achieved by treatment with any np-NTF pool. With regard to neurite lengths, the strongest effect has rather been detected for the single treatment with np-NGF.
Abbreviations: np, nanoparticle; NTFs, neurotrophic factors; DRG, dorsal root ganglion; NGF, nerve growth factor; FGF, fibroblast growth factor; GDNF, glia-derived neurotrophic factor; CTR, control.
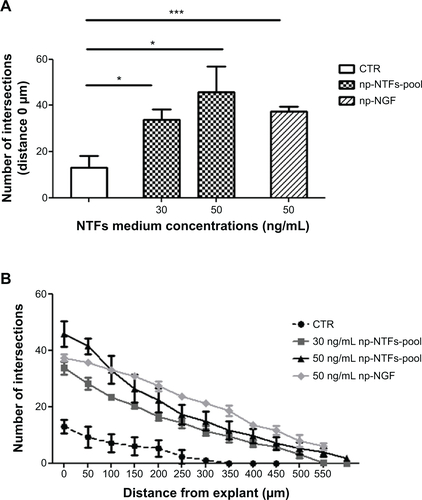
Figure S4 No synergistic effect is induced by supplementing NVR-Gel with a pool of np-NTFs.
Notes: (A) Bar graphs depicting the number of neurite intersections (y-axis) with circles drawn at distinct distances (x-axis) from the neonatal DRG drop culture under control conditions or treatment with 50 ng/mL np-NGF or the 50 ng/mL np-NTF pool. No statistically significant difference between the neurite inductive potential of np-NGF and np-NTF pool was detectable (*P≤0.05, **P≤0.01, ***P≤0.001). (B) Bar graph illustrating the mean length ± standard deviation of the ten longest neurites extending from each of the DRG drop cultures. No significant difference was detectable between both treatments.
Abbreviations: np, nanoparticle; NTFs, neurotrophic factors; DRG, dorsal root ganglion; NGF, nerve growth factor; CTR, control.