Figures & data
Table 1 Glanzmann Thrombasthenia in All Its Forms
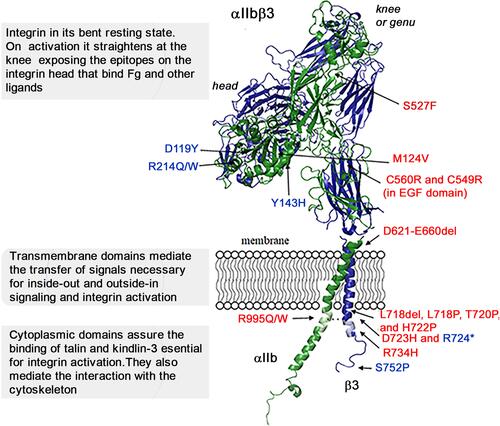
Figure 1 Structural representation of αIIbβ3 in its bent conformation showing the mutations that give rise to selected variant forms of GT or to related phenotypes. This model is based on the crystal structure of αIIbβ3; it was constructed using the PyMol Molecular Graphics System, version 1.3 Schrödinger, LLC and 3fcs and 2knc pdb files as described.Citation16 The αIIb subunit is in green and β3 is in blue. Precision crystallography and modeling showed that αIIb has 4 major extracellular domains (β-propeller, thigh, calf-1 and calf-2) whereas β3 has more (β-I or β-A, hybrid, plexin-semaphorin-integrin (PSI), 4 epidermal growth factor (EGF) and the β-tail domain).Citation8,Citation10 Loss-of-function mutations (in blue) in the β3 extracellular head prevent binding of Fg or other adhesive proteins to the opened integrin headpiece following platelet activation, while those in the β3 cytoplasmic tail prevent binding of kindlin-3 and/or talin, and block steps essential for integrin activation. Gain-of-function mutations (in red) lead to at least partial activation of αIIbβ3 and often associated with MTP accompanied by a variable loss of αIIbβ3 function. All mutations are detailed and referenced in the text.