Figures & data
Figure 1 Timeline of the application of NAT in cancers. Neoadjuvant therapy (NAT) has a long history of being used in the treatment of solid tumors. The commonly used types of NAT are radiotherapy, hormone therapy, chemotherapy, and physical energy therapy. In the 1990s, the outcomes of the initial application of NAT in the treatment of HCC were not satisfactory, leading to absence of large scale application of NAT in HCC treatment.
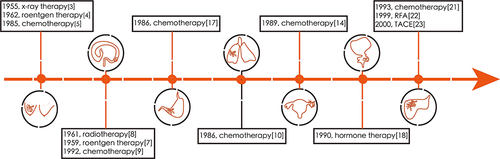
Figure 2 Diagram showing the rationale for NAT in HCC. The occurrence and development of HCC are driven by intrinsic and extrinsic factors. The intrinsic factors include genomic alterations, epigenetic modifications, and abnormal regulation of cell signaling pathways. For these factors, targeted agents, adeno-associated virus-driven gene editing, DNA methyltransferases, and histone deacetylases are being investigated in a NAT setting for HCC. The extrinsic factors primarily comprise interactions between the tumor immune microenvironment and cancer cells. Progression involves three stages. During the elimination stage, tumor neoantigens elicit an immune response that eliminates most malignant cells. During the equilibrium stage, tumor cells with neoantigens that are incapable of inducing an immune response or that have acquired the ability to evade the immune system survive and proliferate. During the escape stage, tumor cells escape immunosurveillance and lead to the development of an immunosuppressed environment. For the target site of the immune cells, an increasing number of agents, such as those that target PD-1/PD-L1, cytotoxic T-lymphocyte antigen 4 (CTLA-4), TGF-β, and TIM-3, have been explored as NATs for HCC.
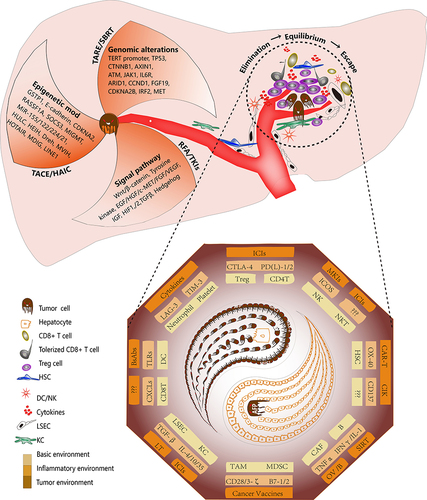
Table 1 Clinical Trials Investigating Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as NATs for HCC
