Figures & data
Figure 1 The mechanism of suppressive immune cells in the TME to promote HCC formation and the potential targets in these cells for HCC immunotherapy. (A) MDSCs suppress CD8+ T, CD4+ T, and NK cells, and stimulate TAMs and Treg cells by releasing various immunosuppressive molecules. (B) TAMs suppress CD8+ T and NK cells, and stimulate MDSC, Treg and HCC cells by releasing various immunosuppressive molecules and expressing a variety of immune checkpoints on the surface. (C) Treg cells suppress CD8+ T and CD4+ T cells by releasing various immunosuppressive molecules and expressing a variety of immune checkpoints on the surface.
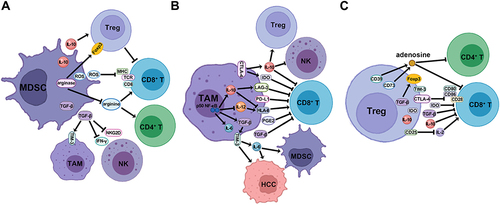
Figure 2 The mechanism of stimulatory immune cells to inhibit HCC formation and the cross-talk with suppressive immune cells in the TME. (A) CD8+ T cells function as an effector, activated by APC through the engagement of co-stimulatory surface molecules, to inhibit HCC cells by releasing cytotoxic molecules and pro-inflammatory cytokines. The activity of CD8+ T cells is dampened by the expression of immune checkpoints on the surface. (B) CD4+ TH1 cells are stimulated by DCs via the binding of co-stimulatory molecules, and inhibited by MDSCs and HCC cells through the expression of immune checkpoints, and reprogrammed to CD4+ TH2 phenotype via the induction of anti-inflammatory cytokines. (C) NK cells suppress HCC cells via releasing a variety of cytotoxic molecules and pro-inflammatory cytokines, and are abrogated by TAM, MDSC and Treg cells via the secretion of anti-inflammatory cytokines, and by cancer cells via the expression of immune checkpoints.
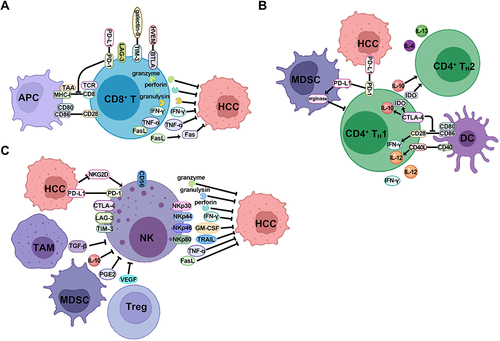
Figure 3 The mechanism of stromal cells in the TME to promote HCC formation. (A) CAFs promote the proliferation of HCC cells, recruit a variety of suppressive immune cells including TAM, MDSC, and Treg cells, and also inhibit the activity of NK, DC and Nt cells through the expression of various cytokines and chemokines. (B) HSFs promote the proliferation of HCC cells through the multiple TGF-β signaling pathways, and recruit MDSC and Treg cells through the release of cytokines and chemokines. (C) LECs conduct antitumor effect by releasing CXCL16 to stimulate the activity of NKTs, and are reprogrammed to inhibit CD8+ T cells when interacting with HCC cells to recruit Treg cells, release immunosuppressive cytokines and express immune checkpoints. (D) DCs act as the APCs to stimulate CD8+ T and CD4+ TH1 cells immune response to HCC, while the subtype of CD4+ DCs suppress CD8+ T cells via the expression of CTLA-4 and immunosuppressive cytokines. KCs serve as another type of APCs to stimulate Treg cells through the secretion of IL-10 and TGF-β, which inhibit the immune stimulation of DCs.
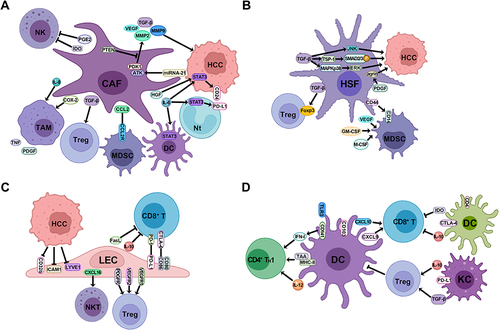
Table 1 The Modification of Immune Components in the TME in Clinical Trials
