Figures & data
Figure 1 Design and construction of the recombinant IAV-OX40L virus. (A) Schematic diagram of the recombinant plasmid. (B) Agarose gel electrophoresis confirmed that the size of the recombinant plasmid was consistent with expectations. (C) Fragment sizes of the eight plasmids were confirmed by agarose gel electrophoresis.
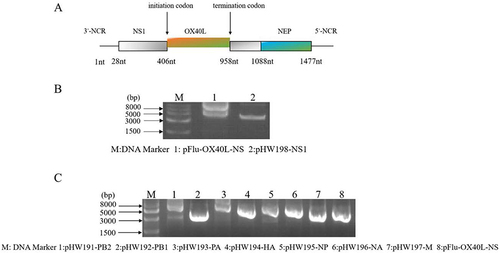
Figure 2 Characterization of the IAV-OX40L virus. (A) The recombinant virus was repeatedly subcultured and the hemagglutination titer of P5 was stabilized at 27–8. (B) The virulence of IAV-OX40L increased gradually during continuous culture and ultimately reached 7–8 LogTCID50/mL. (C and D) The morphology and size distribution of the virus were examined with transmission electron microscopy. (E) The NS fragment was amplified by RT-PCR and identified via agarose gel electrophoresis. (F) Expression of the OX40L protein was confirmed in MDCK and HepG2 cells by Western blot. (G) Immunofluorescence staining of IAV-OX40L virus-infected MDCK and HepG2 cells for the influenza NP protein (red), OX40L protein (green), and DAPI (blue).
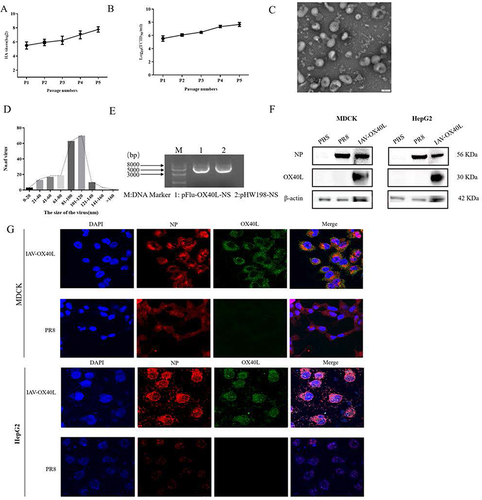
Figure 3 Cytotoxic effects of IAV-OX40L virus in vitro. (A) The growth curve of IAV-OX40L virus was consistent with that of the influenza virus and peaked at 27–8 at 72 h. (B) MTS assays showed that the virus inhibited the growth of tumor cells in vitro in a time- and dose-dependent manner but had no effect on normal cells. (C) Flow cytometry showed that IAV-OX40L significantly promoted the apoptosis of tumor cells, with the most obvious effect at an MOI of 3 but no effect on normal cells. (*P< 0.05, **P< 0.01, ***P< 0.001).
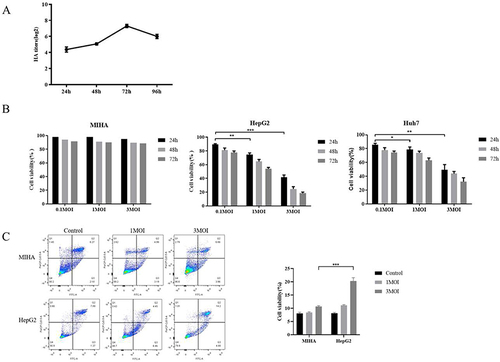
Figure 4 Antitumor efficacy of IAV-OX40L virus in a murine HCC model. (A) The change in tumor volume in the group inoculated with IAV-OX40L virus was significantly slower than in the PR8 and PBS groups. (B–D) Tumor volume and weight in mice in the IAV-OX40L group were significantly smaller than those in the PR8 and PBS groups (6 mice per group). The animal experiment was repeated twice. (E) The viral load in tumor tissues of the IAV-OX40L group was significantly higher than that of the PR8 group, while no viral titer was measurable in other tissues (heart, liver, spleen, lung, kidney, brain) (**P< 0.01).
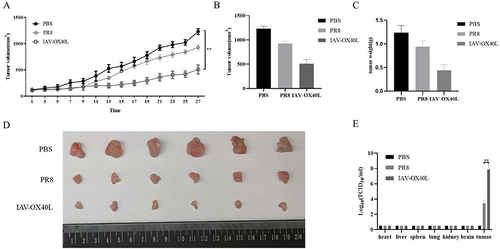
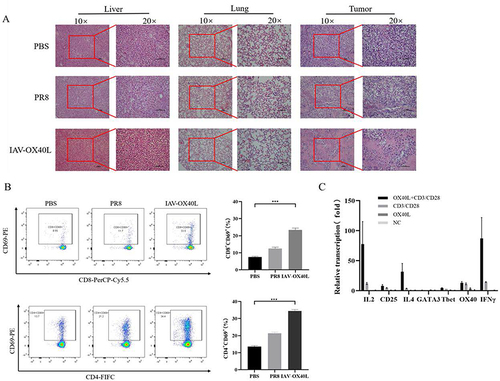
Figure 5 Toxicity and immunological activity induced by IAV-OX40L virus in vivo (A) H&E staining of pathological alterations of liver, lung, and tumor tissues in IAV-OX40L virus-treated mice showed that the virus significantly destroyed tumor tissue without damaging liver and lung tissues. (B) CD4+CD69+ and CD8+CD69+ T cell numbers in the spleen of mice inoculated with IAV-OX40L virus were significantly higher than in the PR8 and PBS groups (***P< 0.001). (C) IAV-OX40L expressed OX40L protein and stimulated CD4 T cells to produce IL-2 and IFN-γ in the presence of CD3/CD28 agonist. CD4 T cells also produces a small amount of T bet and GATA3. Therefore, IAV-OX40L mainly stimulates Th1 immune cells. However, the activation of T cells was also demonstrated by the increase of CD25.