Figures & data
Table 1 The Clinical Baseline Characteristics of Patients in GSE14520
Table 2 The Clinical Baseline Characteristics of Patients in the External Validation Set
Figure 1 The grouping and inclusion/exclusion of datasets in this study. (A) The description and grouping of GSE104580. (B) After applying the exclusion criteria, group GSE14520 was based on the treatment approach. (C) The description and inclusion/exclusion of external validation set.
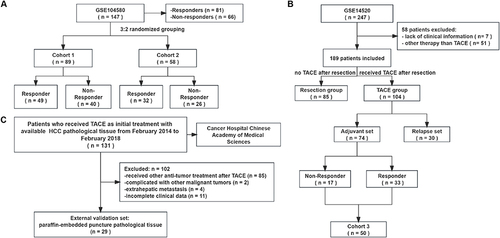
Figure 2 Screening of differential genes related to the effectiveness of TACE. Differentially expressed genes between responders and non-responders in cohort 1 (A) and cohort 3 (B). (C) The Venn diagram showed the intersection of differential genes related to TACE effectiveness in Cohort 1 and Cohort 3.
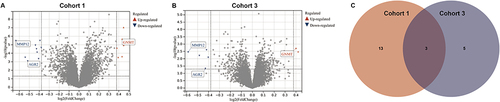
Figure 3 Evaluation of the predictive ability of the TACE effectiveness model by the receiver operating characteristic (ROC) analysis and Kaplan-Meier (KM) analysis. The ROC curves of our model for predicting patients’ response to TACE in cohort 1 (A), cohort 2 (B), and cohort 3 (C). KM analysis of overall survival and disease-free survival between low effectiveness and high effectiveness groups in relapse set (D), adjuvant set (E and F), and TACE group (G).
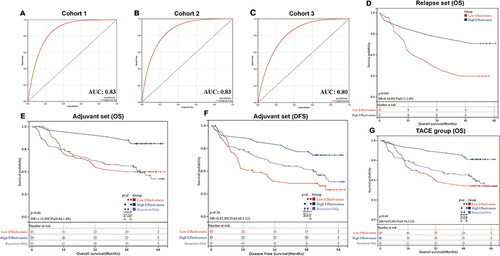
Figure 4 Evaluation of the prediction performance of our model by Kaplan-Meier (KM) analysis, principal component analysis, and survival heat map. KM analysis of overall survival and disease-free survival between low and high effectiveness groups in resection group (A, B). The survival heatmap displayed the distribution of patients’ effectiveness score, prognostic performance, and the expression of signature genes in the TACE group (C), adjuvant set (D), and relapse set (E). The principal component analysis of patients in cohort 1 (F), cohort 2 (G), GSE104580 (H), and TACE group (I) is based on the TACE effectiveness model.
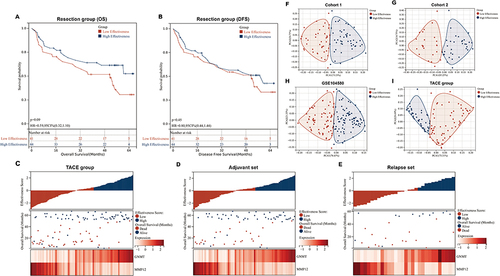
Figure 5 Univariate and multivariate analysis to evaluate the factors influencing the effectiveness of TACE. Univariate analysis to evaluate the factors influencing overall survival (OS) and disease-free survival (DFS) of patients treated with TACE in TACE group (A) and adjuvant set (B). The statistically significant factors identified through univariate analysis were included in the multivariate analysis to assess the independent risk factors influencing OS and DFS in patients treated with TACE in the TACE group (C) and adjuvant set (D). Bolded text indicates statistical significance (p < 0.05).
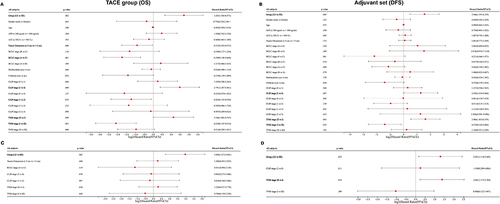
Figure 6 Immunohistochemical (IHC) staining analysis on external validation set to validate the expression trends of the signature genes. (A) Hematoxylin eosin staining and the IHC staining analysis of GNMT and MMP12 in high and low effectiveness group patients. The difference of GNMT (B) and MMP12 (C) relative expression levels between high and low effectiveness group patients. (* p < 0.05; *** p < 0.001).
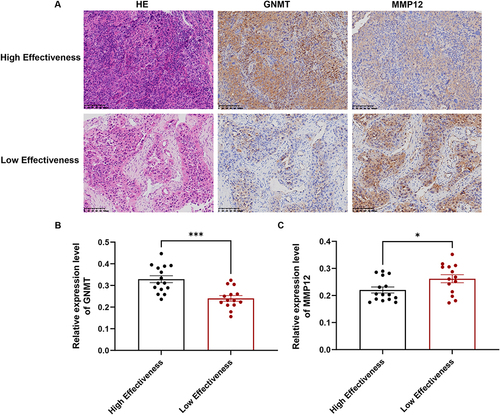
Figure 7 Validation for the predictive ability of the TACE effectiveness model by Kaplan-Meier (KM) analysis and multivariate analysis in external validation set. KM analysis of overall survival (A) and progression-free survival (B) in external validation set. The statistically significant factors identified through univariate analysis were included in the multivariate analysis to assess the independent risk factors influencing overall survival (C) and progression-free survival (D) in patients undergoing TACE in an external validation set. Bolded text indicates statistical significance (p < 0.05).
Figure 8 Immune infiltration and immune simulation analysis to evaluate the infiltration levels of various immune cells and the efficacy of immunotherapy between different groups. The immune infiltration analysis showed the differential infiltration of various immune cells between the low and high effectiveness group in TACE group (A), the non-responder and responder groups in GSE104580 (B), low and high effectiveness group in GSE104580 (C). The immune simulation analysis showed the difference in various immune scores between the low and high effectiveness groups in the TACE group (D), non-responder and responder groups in GSE104580 (E), and LE and HE group in GSE104580 (F). TIDE, tumor immune dysfunction, and exclusion; IFNG, interferon-gamma; MSI, microsatellite instability; MDSC, myeloid-derived suppressor cells; CAF, cancer-associated fibroblasts; TAM, tumor-associated macrophages; LE, low effectiveness; HE, high effectiveness; Re, responder. (* p < 0.05; ** p < 0.01; *** p < 0.001).
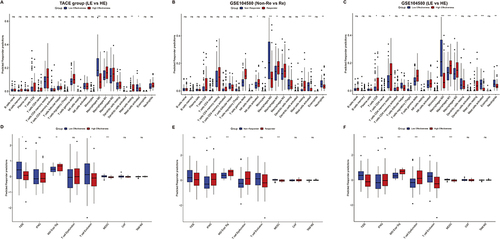
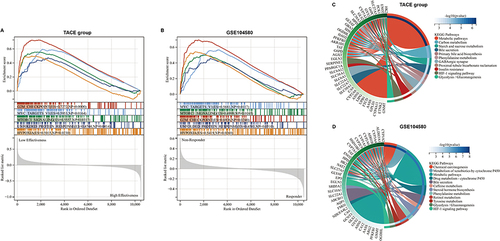
Figure 9 Exploration for related pathways influencing the effectiveness of TACE by functional enrichment analysis. Gene set enrichment analysis exhibited that five hallmark pathways were all enriched in the low effectiveness patients in the TACE group (A) and non-responders in GSE104580 (B). Kyoto Encyclopedia of Genes and Genomes enrichment analysis exhibited that differentially expressed genes between low and high effectiveness groups in TACE (C), non-responder, and responder groups in GSE104580 (D) were enriched in several same pathways.