Figures & data
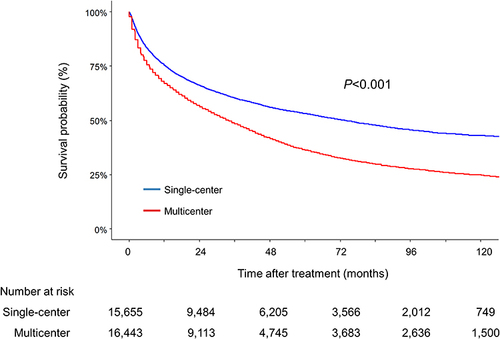
Figure 1 Patient flowchart of the study population.
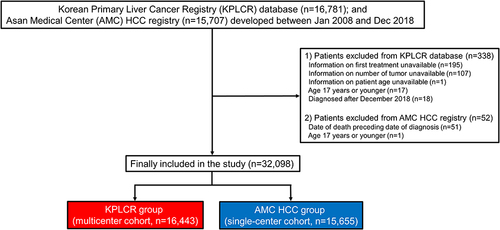
Table 1 Baseline Characteristics of the Study Populationsa
Table 2 Cox Regression Analysis of Factors Associated with Mortality in the Entire Cohortsa
Table 3 Cox Regression Analysis of Risk of Mortality by Initial Treatment in the Entire Cohortsa
Table 4 Cox Regression Analysis of Risk of Mortality by Initial Treatment in BCLC-Guided Subcohortsa
Figure 3 Kaplan–Meier estimates of overall survival of patients who received (A) surgical resection, (B) liver transplants, (C) LAT, (D) TACE, and (E) systemic therapy according to the treatment indications*.
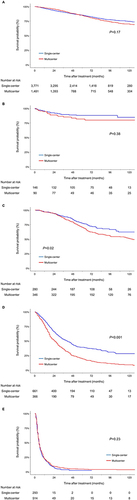
Table 5 Subgroup Analysisa