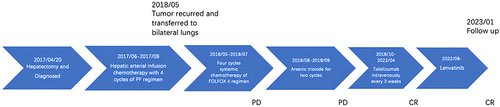
Figures & data
Figure 1 (A) The huge lump on the right lobe of the liver was surgically removed in April 2017. (B) The postoperative follow-up (May 2018) showed tumor recurrence in the lungs. (C) At the end of the last arsenic trioxide chemotherapy and before the use of tislelizumab (October 2018). (D) The efficacy was first evaluated as a partial response after 2 cycles of treatment with tislelizumab (January 2019). (E) The efficacy was first evaluated as a complete response after 12 cycles of treatment with tislelizumab (July 2019). (F) The patient still had a complete response at the latest follow-up (July 2023). The red arrow in indicates the location of the primary tumor. Arrows in indicate the location of recurrent tumors in the lungs.
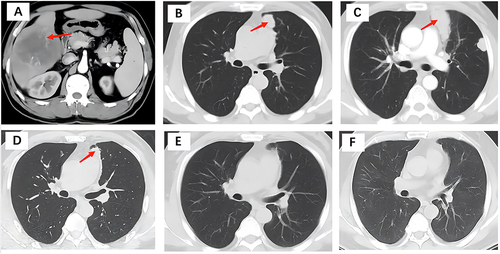
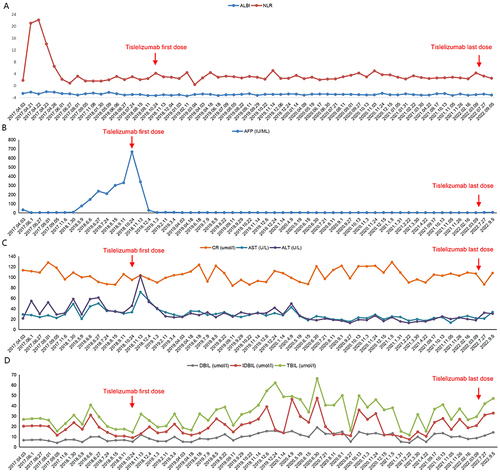
Figure 2 Evolution in time of the albumin-bilirubin (ALBI) score and neutrophil-to-lymphocyte ratio (NLR) (A), α-fetoprotein (AFP) levels (B), aspartate aminotransferase (AST), creatinine (CR), and alanine aminotransferase (ALT) (C), direct bilirubin (DBIL), indirect bilirubin (IDBIL), and total bilirubin (TBIL) (D).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon a reasonable request.