Figures & data
Figure 1 The IgG subtypes.
Notes: Four subtypes of IgG exist in humans: IgG1, IgG2, IgG3, IgG4, each with differences in sequence, structure, glycosylation and communication with FcγRs. The four subtypes are named based on their respective abundance in serum with IgG1 being the most abundant. IgG antibodies consist of two Fab regions that can bind both an antigen molecule and the Fc region which interact with the FcγR, joined by a highly flexible hinge region. IgG has a longer hinge region than the other IgG subtypes. Each IgG subtype has conserved Asn 297 amino acids in the Fc region with N-glycans attached (shown in yellow). Typically, the Fc glycans are bi-antennary galactosylated structures with varying amounts of core fucosylation and sialylation. Glycosylation is also found in the Fab regions with higher proportions of galactosylated and sialylated glycans. Heavy chains are shown in green, and light chains are shown in blue and purple.
Abbreviations: Fab, fragment antigen binding; FcγR, Fc gamma receptor; IgG, immunoglobulin G.
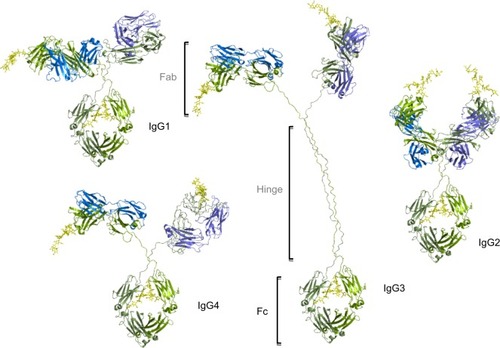
Table 1 Properties of human FcγRs
Table 2 Annotation of human FcγR glycosylation showing position and conservation of N-glycan sites
Figure 2 Human FcγRs are complex glycoproteins.
Notes: FcγR ectodomains were modeled with N-glycans on each glycosylation site for each receptor, taking into account glycan size and composition, torsion angles and free energies. The N-glycans (shown in cyan) were modeled based on identified glycans from FcγRs (shown in gold) produced in recombinant systems such as NS0, CHO and HEK293 systems.Citation43,Citation47,Citation50 In the case of FcγRIIIa, site-specific glycosylation studies were performed by Zeck et al,Citation47 and this information was used to build the glycans shown on the Asn 162 site located at the binding interface with IgG. For the remaining receptors, no site-specific analysis was available, and in these cases the most abundant glycans identified from the recombinant sources were used to model N-glycosylation for these receptors. FcγRI has an extra D3 domain, which contributes to its high-affinity nature, and this domain also contains two glycosylation sites. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the Oxford notation (https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcγRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcγRIIa: Asn 64 (FA2G2S1), Asn 145 (FA2BG2). FcγRIIb: Asn 66 (FA2G2S1), Asn 147 (FA2BG2). FcγRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). FcγRIIIb: Asn 35 (FA2GalNAc2S2), Asn 42 (Man 5), Asn 61 (FA2G2S1), Asn 71 (FA3G2), Asn 159 (FA2BG1), Asn 166 (FA2BG2). PDB accession numbers used to build the models were as follows: FcγRI: 4×4m, FcγRIIa: 1fcg, FcγRIIb: 2fcb, FcγRIIIa: 3ay4, FcγRIIIb: 1e4j.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G; PDB, Protein Data Bank.
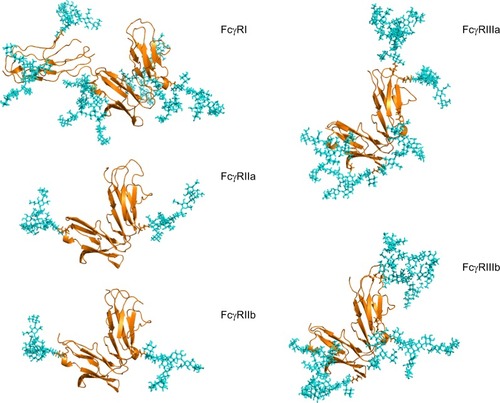
Figure 3 FcγR–IgG complexes with modeled N-glycans show complexity of glycosylation and the potential roles of glycans in the binding interaction with IgG.
Notes: The Asn 162 glycan of FcγRIIIa has been shown to form carbohydrate–carbohydrate interactions with the bi-antennary glycan of IgG.Citation40 This asparagine residue is at the binding interface with IgG. FcγRI does not have a glycan in this position but does have glycans near the binding site such as Asn 78 (Asn 162 in FcγRIIIa), which is structurally conserved in each of the FcγRs. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the oxford notation (see https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcgRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcgRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). PDB accession numbers used to build the models were as follows: FcgRI: 4×4m, FcgRIIIa: 3ay4.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G.
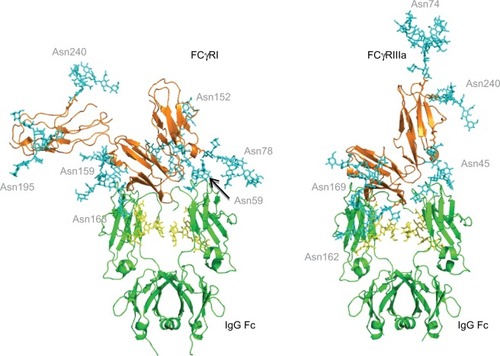
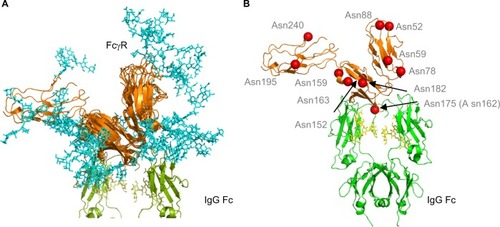
Figure 4 Human FcγRs show complex N-glycosylation in the protein ectodomain and around the IgG binding site.
Notes: (A) Structural overlay of FcγRs. FcγRs are very structurally homologous with the exception of the extra D3 domain in FcγRI. Complexity of glycans (cyan) is shown and potential interactions with IgG Fc and the IgG Fc glycans. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the oxford notation (see https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcgRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcgRIIa: Asn 64 (FA2G2S1), Asn 145 (FA2BG2). FcgRIIb: Asn 66 (FA2G2S1), Asn 147 (FA2BG2). FcgRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). FcgRIIIb: Asn 35 (FA2GalNAc2S2), Asn 42 (Man 5), Asn 61 (FA2G2S1), Asn 71 (FA3G2), Asn 159 (FA2BG1), Asn 166 (FA2BG2). PDB accession numbers used to build the models were as follows: FcgRI: 4×4m, FcgRIIa: 1fcg, FcgRIIb: 2fcb, FcgRIIIa: 3ay4, FcgRIIIb: 1e4j. (B) Position of N-glycan sites in human FcγRs. Asn 175 (Asn 162 in FcγRIIIa) is at the binding site with IgG and glycans in this position can participate in carbohydrate–carbohydrate interactions. A number of other glycan sites are present close to the binding interface with IgG and glycans in this position can potentially participate in glycan–glycan interactions and glycan–protein interactions with IgG Fc. Structure is based on FcγRI (PDB: 4×4m). N-glycosylation sites are named for FcγRI using the UniprotKB numbering scheme.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G; PDB, Protein Data Bank.