Figures & data
Figure 1 Activation of caspase-1 and its role in the initiation of inflammation. Different types of inflammasome can be activated by their distinct stimuli’s. Of these, NLRP3 can be activated by several PAMPs and DAMPs that enter into the cytosol through TLRs or phagocytosis. Moreover, in response to extracellular ATP, efflux of K+ from intracellular via P2X7 and release of Ca++ from storage site called smooth endoplasmic reticulum via Ca2+ channel leads to mitochondrial dysfunction and generation of ROS could trigger oxidative stress, which can then activate NLRP3 inflammasome. All inflammasomes finally activate procaspase-1 to caspase-1, and in turn matures pro-IL-1β and pro-IL-18 to induce inflammatory response. Caspase-1 activated gasdermin-D serves as pore-forming protein channels for IL-1β and IL-18 secretion, then high abundance of gasdermin-D at the plasma membrane results in inflammatory-induced lytic form of cell death called pyroptosis. NLRC4 inflammasome does not have ASC/adaptor protein, and the CARD domain of NLRC4 directly binds to its respective domain of procaspase-1.
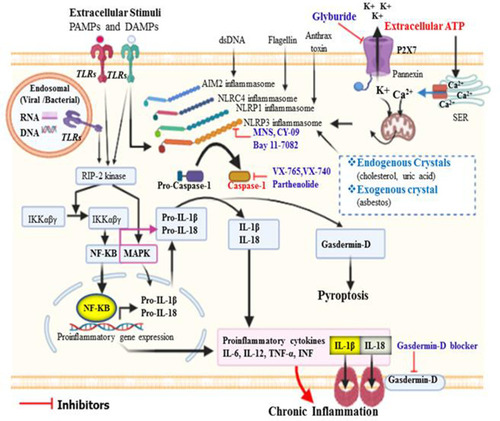
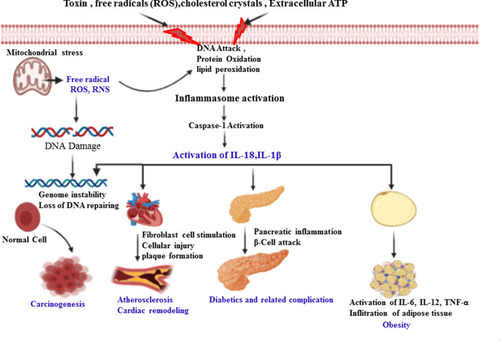
Figure 2 The role of caspase-1 in the pathogenesis of inflammatory-induced NCDs. Activation of caspase-1 by internal/external stimuli matures pro-inflammatory cytokines (IL-1β) and gasdermin-D to induce pyroptosis. This initiates inflammatory response and may result in cell injury and/or death, plaque formation and rupture, infiltration to several organs, in turn leading to the development and progression of obesity, atherosclerosis, cardiac remodeling and cancer formation. The release of ROS/RNS from stressed mitochondria may directly oxidize and damage cellular/extracellular proteins, carbohydrates, lipids and nucleic acids that lead to metabolic disturbance. Thus, DNA damage leads to an alteration of signaling pathways (suppression of tumor suppressor genes and promotion of tumor promoter genes) that further results in exacerbation of DNA damage, genomic instability and impairment of DNA repair system. These collectively may promote carcinogenesis. These metabolic disturbances further result in the production of NLRP3 inflammasome stimulatory molecules such as cholesterol crystals, ROS/RNS, damaged cells and ATP, which in turn activates caspase-1 in positive a loop pathway which aggravates the inflammatory process and infiltration of the inflammatory immune cells.