Figures & data
Table 1 Epidemiology and Common Inflammatory Cytokines in Patients Presenting with SARS-CoV-2,Citation4,Citation14,Citation16,Citation18–Citation24 Compared to SARS-CoVCitation18,Citation23,Citation25–Citation27 and MERS-CoVCitation12,Citation14,Citation18,Citation25,Citation28
Figure 1 Th-17 cell polarization from naïve CD4+ T cells and activation. In response to SARS-CoV-2, antigen-presenting cells (APCs) such as dendritic cells as well as macrophages can present the fragments of the antigen to the naïve CD4+ T cells, and upon activation APCs release IL-6, TGF-β, and IL-23 polarizing cytokines. In turn, IL-6 binds with its receptor and through JAK-STAT3 causes polarization, maturation, and expansion of CD4+ T cells to Th17 cells with the expression of RORγt. In turn, the activated Th-17 cells produce inflammatory cytokines such as IL-17A, IL-17F, IL-21, and IL-22.
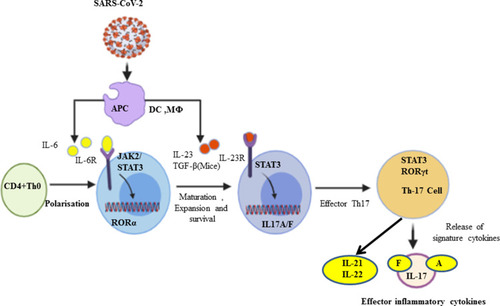
Figure 2 Signaling pathways of IL-17A, pathological effect, and potential drug targets. (A) Signaling transduction of IL-17A at type I epithelial cells of the alveoli of the lung. IL-17A and IL-17F are secreted predominantly by Th-17 cells; they are structurally very similar, bind the IL-17RA–IL-17RC receptor combination, and can form heterodimers together, signaling via the adaptor protein nuclear factor (NF)-κ activator (Act1). Many IL-17 target genes contain a promoter region that binds with NF-κB. IL-17 is not a potent inducer of inflammation by itself. Its strong effects during inflammation are derived from its ability to recruit immune cells via chemokine expression such as CXCL1, CXCL5, and MCP1, as well as from its synergistic action with other cytokines such as IL-6, IL-1, and TNFα. Thus, IL-17, acting in synergy with IL-6 and TNF, is a powerful inflammatory signal that results in the rapid recruitment and sustained presence of neutrophils and leads to a cytokine storm. (B) Schematic representation of IL-17A-mediated immunopathological effect of ARDS during SARS-CoV-2 infection. Pulmonary fibrosis is one of the pathological changes due to the activation of fibroblasts mediated by IL-6 and results in abnormal deposition of collagen. Moreover, the stimulation of fibroblasts can produce IL-8 and leads to the attraction of neutrophils to the site of injury. MMP3/9 (which causes tissue destruction) and PGE2 (increases capillary permeability) are also responsible for neutrophil infiltration, alveolar edema, and protein-positive (exudative) pleural effusion. Altogether, the proposed pathological mechanism suggests that IL-17 can mediate numerous immunopathological effects in CRS secondary to SARS-CoV-2 infection.
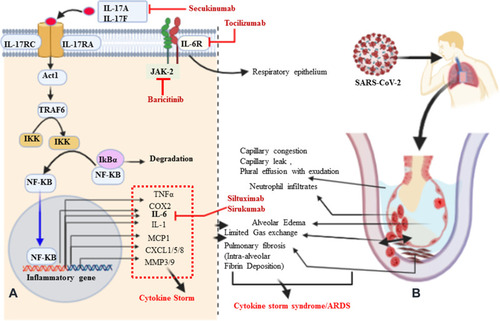
Table 2 Summary of Clinical Trials Investigating the Implications of Anti-Inflammatory Agents in COVID-19 Patients, Registered Under Clinicaltrials.gov, WHO Trial Registry Network, and NIH
