Figures & data
Figure 1 Model of NLRP3 activation. Signals 1 and 2 are both essential for NLRP3 activation. Regarding signal 1, the NF-κB pathway can be activated by TLRs, RLRs and TNF-α signaling, leading to pro-IL-1β and pro-IL-18 upregulation. Regarding signal 2, various PAMPs and DAMPs induce ASC assembly and caspase-1 maturation, which involves NLRP3 activation. In some cases, this is related to the presence of a high concentration of extracellular ATP and subsequent K+, Cl− efflux via P2X purinoceptor 7 (P2RX7) channels, pannexin-1 or CLIC channels. Additionally, nigericin, asbestos, and other particulates can induce lysosomal damage, releasing cathepsin B into the cytosol. Moreover, ROS can activate NLRP3 by inducing Ca2+ influx through transient receptor potential melastatin 2 (TRPM2) channels. The ALRs can interact with NLRP3 leading to pro-IL-1β and pro-IL-18 upregulation. Lastly, NLRP3 can bind to the dispersed trans-Golgi network (dTGN) via PI4P. Downstream of these processes, IL-1β and IL-18 are released.
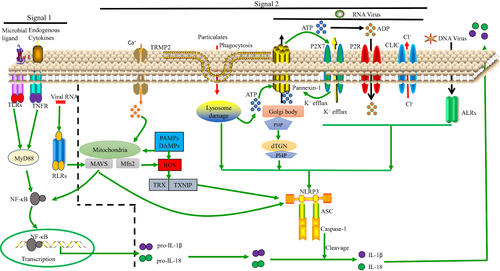
Figure 2 Various RNA viruses can activate NLRP3 inflammasomes. EV-71, Zika virus (ZIKV), influenza virus, and hepatitis C virus (HCV), DENV NS2A, NS2B use the ROS-dependent pathway to form the NLRP3 inflammasome complex. Influenza virus can also rupture lysosomes and then activate NLRP3 inflammasomes via a cathepsin B-dependent process. SeV uses MAVS, and EMCV uses mitofusin-2 to activate NLRP3. FMDV 2B, HRV 2B, and EMCV 2B proteins activate NLRP3 via the Ca2+ model, while HCV viral RNA, RSV viral RNA, Sars-CoV 3A and influenza M2 use the K+ model involving pannexin channels. Influenza M2 and RSV also use the Golgi network to activate NLRP3. Various RNA viruses can inhibit NLRP3 inflammasomes using virus-encoded proteins. The influenza NS1 protein prevents caspase-1 activation and impairs the transcription of pro-inflammatory cytokines to prevent IL-1β and IL-18 release. The influenza A PB1-F2 protein impairs mitochondria in order to inhibit NLRP3 activation. The measles virus (MV) V protein prevents the transcription of IFN-α/β. The SeV V protein prevents NLRP3-mediated ASC oligomerization. The EV-71 2A and 3C proteases directly cleave NLRP3.
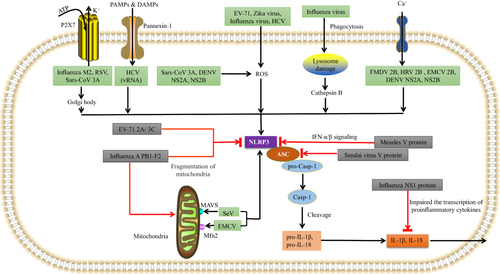
Table 1 Summary of the Components of RNA Viruses That Affect the NLRP3 Inflammasome
