Figures & data
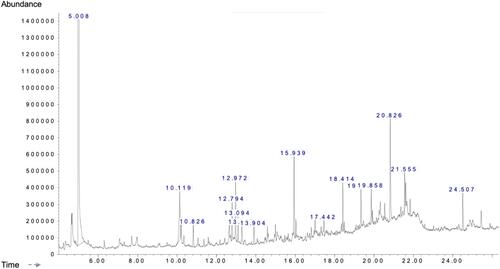
Table 1 Biologically Active Chemical Compounds of FSE by GC-MS Analysis
Figure 2 Concentration of polyphenols in FSE and antioxidant activity.
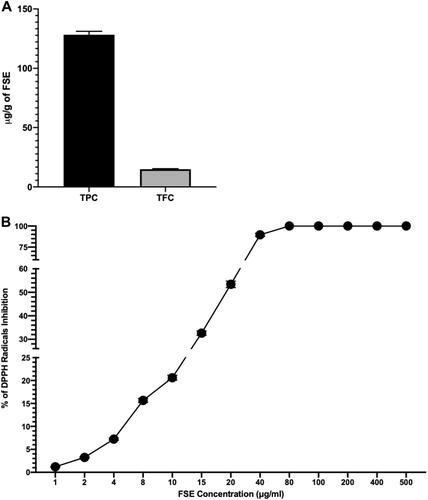
Figure 3 The analysis of cell viability by cell cytotoxicity assay.
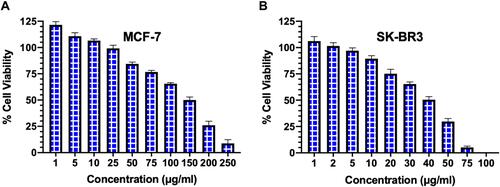
Figure 4 FSE induced morphological changes in breast cancer cells.
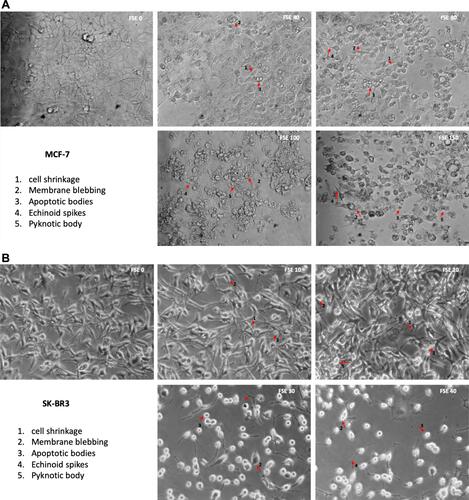
Figure 5 FSE inhibited metastatic properties of breast cancer cells by wound healing/scratch motility assay.
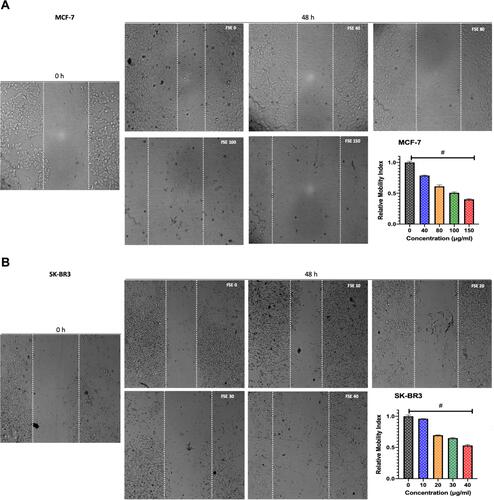
Figure 6 Effect of FSE on adhesion of breast cancer cells by crystal violet staining assay.
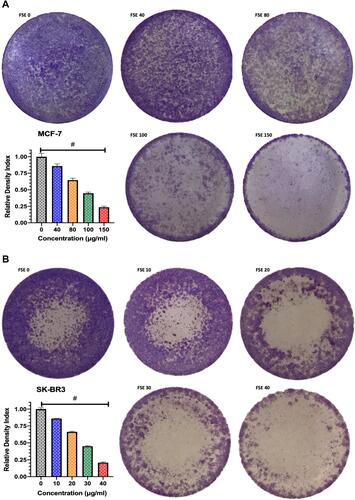
Figure 7 FSE induced apoptosis using Annexin V-FITC/PI by flow cytometry.
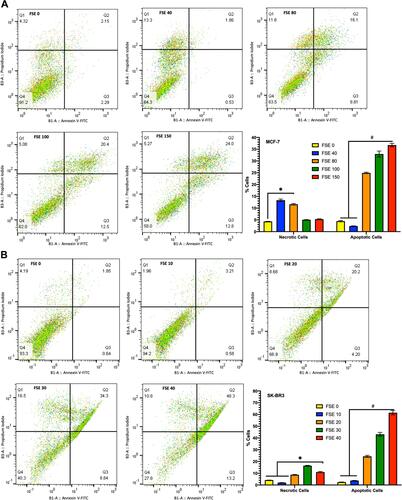
Figure 8 Effect of FSE on cell cycle distribution using PI by flow cytometry.
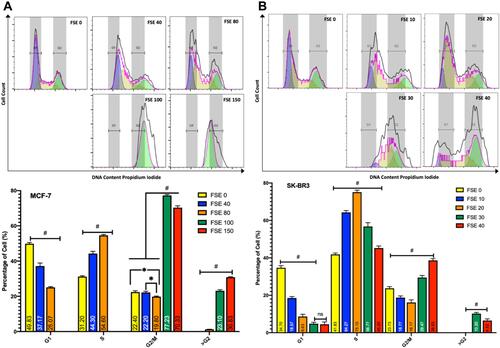
Figure 9 Effect of FSE on mitochondrial membrane potential (MMP or ΔΨm). (A) MCF-7 and (B) SK-BR3 cells (2.5 × 105 cells for flow cytometry and 5 × 104 cells for confocal microscopy) were grown, followed by the treatment with the specified concentrations for 48 hours in 6 well and 24 well plates, respectively. The ΔΨm of the cells was measured quantitatively and qualitatively using TMRE by the flow cytometry and confocal microscopy (Magnification, 300X), correspondingly. The representative images and analyses of ΔΨm as ± SEM of three independent experiments. Statistical differences were analyzed by Ordinary one-way ANOVA, Tukey’s multiple comparison test. #Significant difference between each treated group. The FCCP (20 µM) was added 10 minutes prior to staining with TMRE in FSE 0 (+ve Ctrl) treated cells.
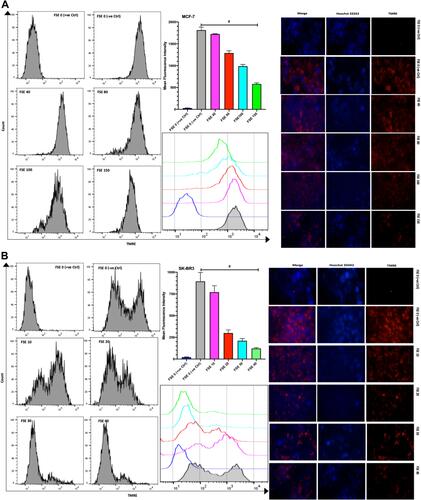
Figure 10 Effect of FSE on the generation of reactive oxygen species (ROS).
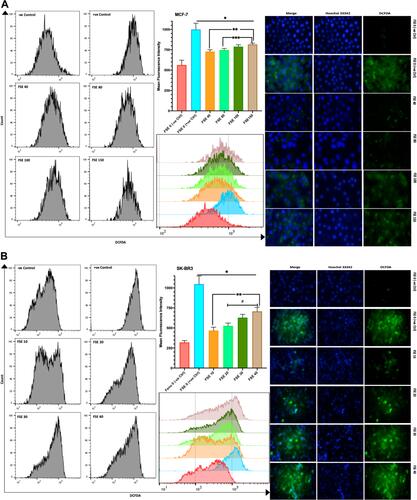
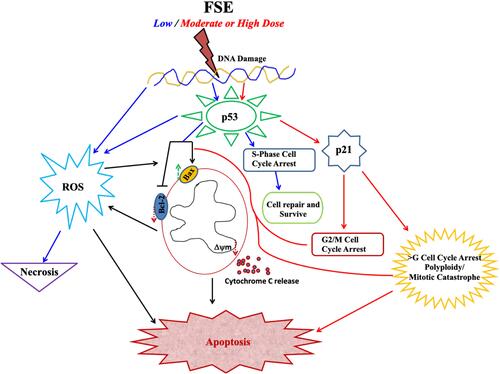
Figure 11 Effect of FSE on p53 signaling using the Western blotting.
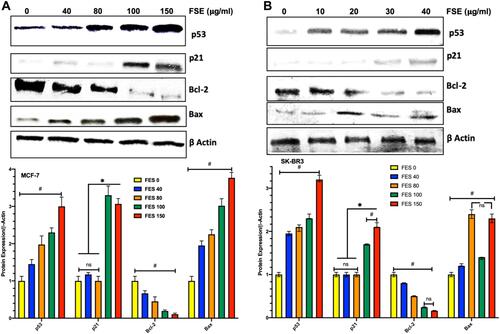
Table 2 General Appearance and Behavioral Observations of Oral Acute Toxicity Study for Vehicle and FSE Treated Groups
Figure 12 Effect of FSE on body weight and relative organs weight (ROW).
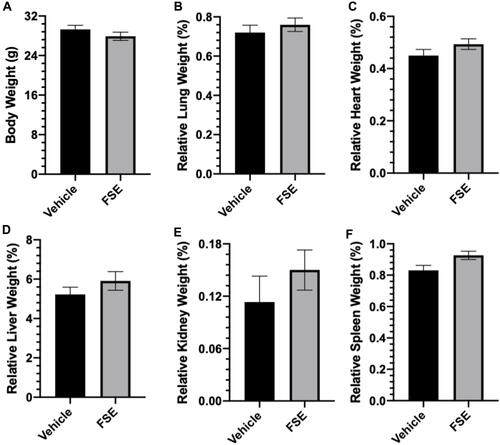
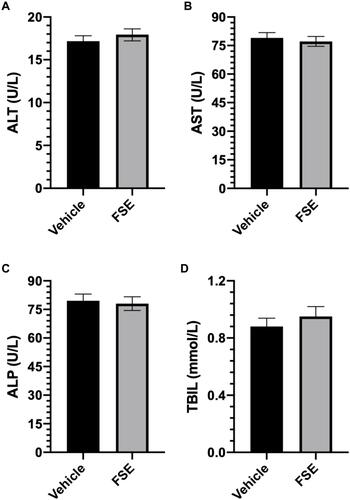
Figure 13 Effect of FSE on biochemical parameters.