Figures & data
Table 1 List of Primers
Figure 1 Time-course of mouse characteristics in wild-type (WT), Pristane and FcGRIIB-/- groups as determined by lupus characteristics (serum creatinine, urine protein and anti-dsDNA) (A–C) and gut permeability defect (FITC-dextran assay) (D) are demonstrated (n = 5–7/time-point). Additionally, the parameters of mice at 40 wks old, including gut translocation of serum lipopolysaccharide (endotoxemia) and serum (1→3)-β-D-glucan (BG) (E and F), systemic inflammation (serum IL-6) (G) and neutrophil extracellular traps (NETs) in glomeruli using immunofluorescent staining of myeloperoxidase (MPO) (red color of Alexa Fluor 647) and neutrophil elastase (NE) (green color of Alexa Fluor 488) as indicated by the percentage of mean fluorescent intensity (MFI) (H and I) and the representative pictures (J) are demonstrated (n = 7–9/groups for E-I). *p < 0.05.
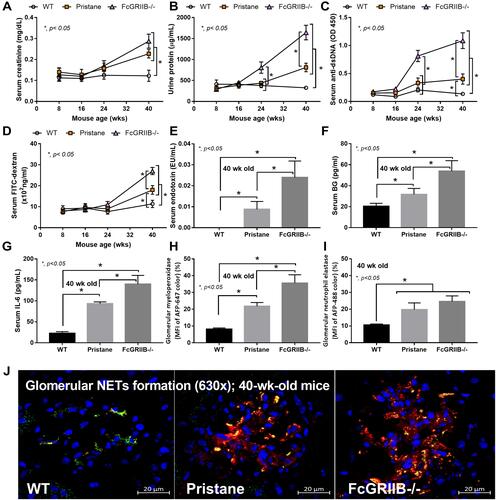
Figure 2 Characteristics of dextran sulfate solution (DSS) administered mice from wild-type (WT), Pristane and FcGRIIB-/- group as determined by body weight alteration in time-points (A–C) and at 7 days post-DSS (D) together with stool consistency index in time points (E–G) and at 7 days post-DSS (H) are demonstrated (n = 6–9/time-point or group). *p < 0.05 vs water; **p < 0.001 vs water; #P < 0.05 vs DSS; ##p < 0.001 vs DSS; ϕp < 0.05 vs WT + water.
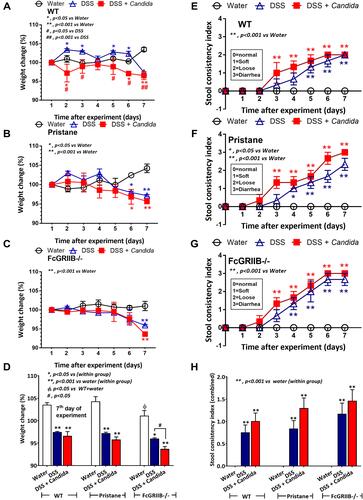
Figure 3 Characteristics of colon injury in mice from wild-type (WT), Pristane and FcGRIIB-/- groups after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS+Candida) as determined by colon injury score (A), colon immunofluorescent stains for neutrophils (Ly6G), immunoglobulin G (IgG) deposition, myeloperoxidase (MPO) and neutrophil elastase (NE) (B–E) and gene expression by polymerase chain reaction (PCR) for PAD4 and tissue cytokines (IL-1β, IL-6 and TNF-α) (F–I) are demonstrated (n = 6–9/group). *p < 0.05 vs water (within group); **p < 0.001 vs water (within group); ϕp < 0.05 vs WT + DSS; δp < 0.05 vs WT + DSS + Candida; #p < 0.05.
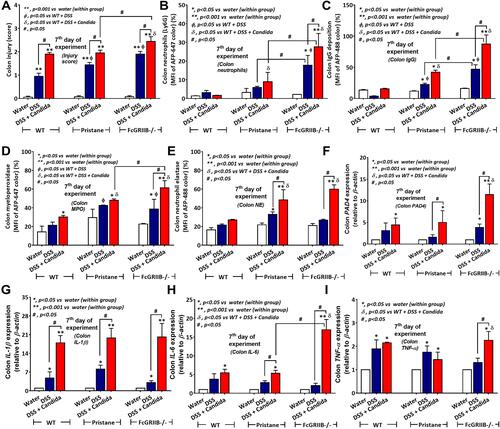
Figure 4 Representative pictures of hematoxylin and eosin (H&E) stained sections from colons of mice from wild-type (WT), Pristane and FcGRIIB-/- groups after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS+Candida) and FcGRIIB-/- with water control (water) (original magnification 200x) are demonstrated. A high magnification (400x) picture at the ulcer-base in FcGRIIB-/- mice with DSS + Candida (the inset picture with blue outline) indicates neutrophil infiltration in the colon ulcer. Colon pictures of mice with control water from WT and Pristane group are not presented due to the similarity to FcGRIIB-/- with water control. Red arrows indicate ulcer lesions.
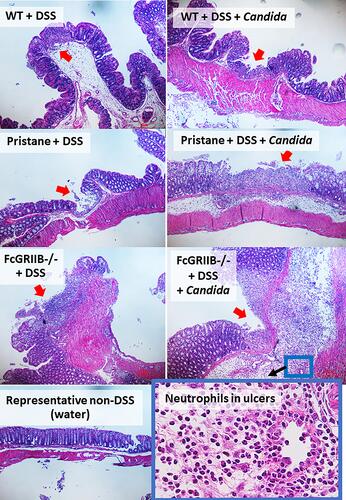
Figure 5 Representative pictures of immunofluorescent stained sections for colon injury as determined by immunoglobulin G (IgG deposition) (green color of Alexa Fluor 488) and neutrophil accumulation (Ly6G) (red color of Alexa Fluor 647) of mice from wild-type (WT), Pristane and FcGRIIB-/- group after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS + Candida) (original magnification 630x) are demonstrated. Colon pictures from mice with control water in WT, Pristane and FcGRIIB-/- group are not presented due to the similarity to the represented pictures of WT+DSS and WT+DSS+Candida.
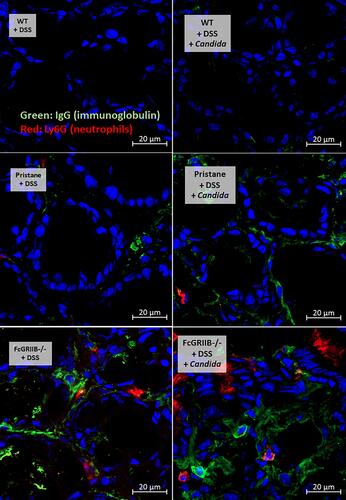
Figure 6 Representative picture of immunofluorescent stained sections for neutrophil extracellular traps (NETs) in colons as determined by neutrophil elastase (NE) (green color of Alexa Fluor 488) and myeloperoxidase (MPO) (red color of Alexa Fluor 647) of mice from wild-type (WT), Pristane and FcGRIIB-/- groups after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS + Candida) (original magnification 630x) are demonstrated. Colon pictures from mice with control water in WT, Pristane and FcGRIIB-/- group are not presented due to the similarity to the represented pictures of WT+DSS.
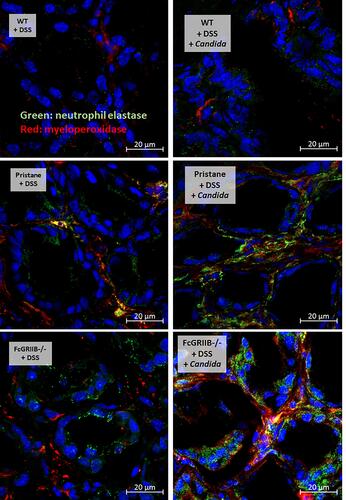
Figure 7 Characteristics of systemic impact on colon injury in mice from wild-type (WT), Pristane and FcGRIIB-/- groups after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS+Candida) as determined by gut permeability defect (gut-leakage) including FITC-dextran assay, endotoxemia and serum (1→3)-β-D-glucan (A–C) and systemic inflammation as indicated by peripheral blood neutrophils (D), serum dsDNA (a marker of neutrophil extracellular traps) (E), serum cytokines (IL-6, TNF-α and IL-10) (F–H) and anti-dsDNA (a major lupus auto-antibody) (I) are demonstrated (n = 6–9/group). *p < 0.05 vs water (within group); **p < 0.001 vs water (within group); ϕp < 0.05 vs WT+DSS; δp < 0.05 vs WT + DSS + Candida; #p < 0.05.
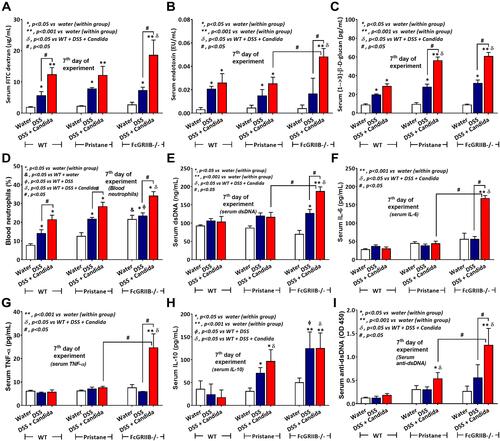
Figure 8 Characteristics of renal injury in FcGRIIB-/- mice after the administration of dextran sulfate solution (DSS) alone or with Candida gavage (DSS+Candida) as determined by serum creatinine (A), urine protein (B), glomerular injury (mesangial expansion) from the histology (C), immunoglobulin G (IgG) deposition in glomeruli (D) and glomerular neutrophil extracellular traps (NETs) as indicated by immunofluorescence of myeloperoxidase (MPO) (red color of Alexa Fluor 647) and neutrophil elastase (NE) (green color of Alexa Fluor 488) (E and F) are demonstrated (n = 6–9/group). Additionally, representative pictures of hematoxylin and eosin (H&E) stained section from kidney with immunofluorescence of glomerular IgG deposition (inset pictures) (G) and glomerular NETs formation (MPO and NE) (H) are demonstrated (original magnification 400x for H&E stain and 600x for immunofluorescence). Pictures from WT and Pristane mice are not presented due to the non-difference from FcGRIIB-/- mice with water control. *p < 0.05 vs water (within group); **p < 0.001 vs water (within group); ϕp < 0.05 vs WT+DSS; δp < 0.05 vs WT (or pristane) + DSS + Candida; #p < 0.05.
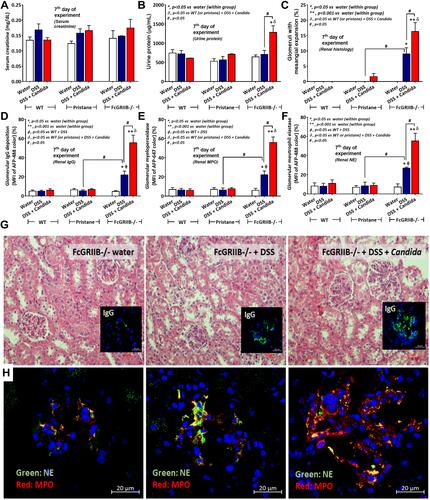
Figure 9 Neutrophil extracellular traps (NETs) in mouse neutrophils, from wild-type (WT) and FcGRIIB-/- mice, and human neutrophils after the 2 h activation by phosphate buffer solution (PBS) or (1→3)-β-D-glucan (BG) or lipopolysaccharide (LPS) or LPS with BG (LPS+BG) are demonstrated. In mouse neutrophils, NETs were determined by the percentage of cells with NETs nucleus morphology using 4-,6-diamidino-2-phenylindole (DAPI), a nucleus stained color, with supernatant dsDNA by PicoGreen assay (A and B). In human neutrophils, NETs using the percentage of cells with positive staining for both myeloperoxidase (MPO) together with neutrophil elastase (NE) (merge color) (C) and the supernatant dsDNA (D) are demonstrated. All experiments were independently performed in triplicate. *p < 0.05 vs WT + PBS; **p < 0.001 vs WT + PBS; ϕp < 0.05 vs WT + LPS or BG; #p < 0.05.
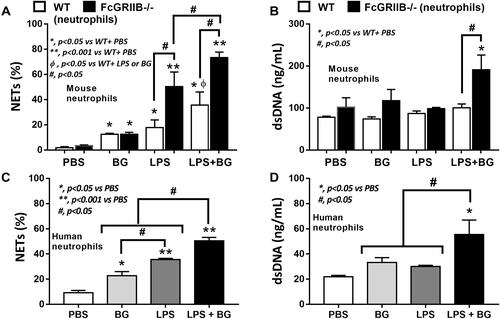
Figure 10 Representative immunofluorescent pictures for neutrophil extracellular traps (NETs) from wild-type (WT) or FcGRIIB-/- neutrophils after the 2 h activation by phosphate buffer solution (PBS) or (1→3)-β-D-glucan (BG) or lipopolysaccharide (LPS) or LPS with BG (LPS+BG) as determined by the branching of nucleus morphology using 4-,6-diamidino-2-phenylindole (DAPI), a nucleus stained color (blue), in mouse neutrophils (upper part) and the co-localization (merge purple color) of myeloperoxidase (MPO) (red) with neutrophil elastase (NE) (green) in human neutrophils (lower part) are demonstrated.
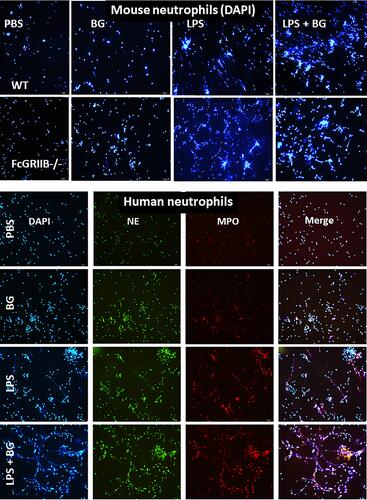
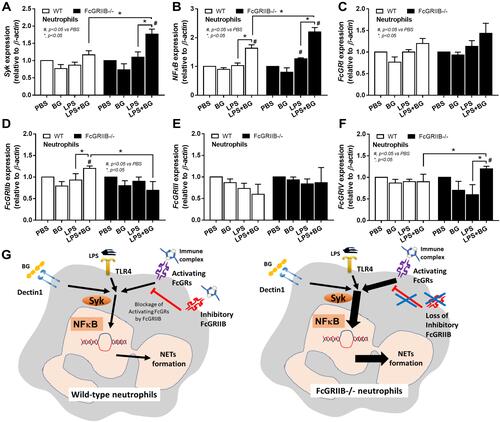
Figure 11 Characteristics of neutrophils from wild-type (WT) and FcGRIIB-/- mice after the 2 h activation by phosphate buffer solution (PBS) or (1→3)-β-D-glucan (BG) or lipopolysaccharide (LPS) or LPS with BG (LPS+BG) as determined by the gene expression of Spleen tyrosine kinase (Syk) (A), Nuclear factor kappa B (NFκB) (B) and activating FcGRs (FcGRI, FcGRIII, FcGRIV) and inhibitory FcGRIIB (C–F) are demonstrated. All experiments were independently performed in triplicate. Additionally, the proposed hypothesis (G) indicates a possible difference in NETs formation between WT and FcGRIIB-/- neutrophils that proposes Syk as a common downstream signaling of TLR-4, Dectin-1 and activating-FcGRs that could be partly blocked by the inhibitory FcGRIIB. #p < 0.05 vs WT + PBS; *p < 0.05.