Figures & data
Figure 1 Role of different regulatory factors in m6A modification of RNA. Writers, erasers, and readers play different roles in the dynamic m6A modification of RNA. Methyltransferase complex with METTL3 as the core positively regulates m6A modification, while demethylases represented by FTO and ALKBH5 negatively regulate m6A modification. Also, the recognition of m6A modification requires the recognition and combination of various readers.
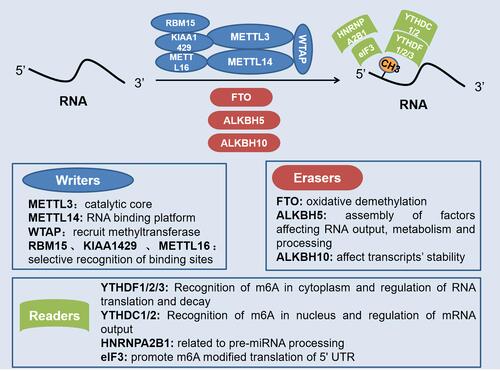
Figure 2 Effects of m6A on the normal maturation and physiological function of DCs. (A) the role of m6A in the maturation of DCs and activation of T cells. The m6A modification of Trap transcription factor in DCs promotes the translation of CD40, CD80, and TLR4 signals on its membrane surface, which not only activates T cells but also enhances the production of cytokines induced by TLR4/NF-κB signals. (B) the role of m6A in the migration of mature DCs. Stimulation of CCR7 activates the HIF-1α transcription factor pathway in DC. m6A modified lnc-Dpf3 directly binds to HIF-1α and inhibits the transcription of HIF-1α-dependent glycolytic gene LDHA, thus inhibiting the glycolytic metabolism and migration ability of DCs. (C) m6A mediated the antitumor effect of DCs. Transcript Flt3L encoding lysosomal protease is labeled with m6A and recognized by YTHDF1, which enhances the translation of lysosomal cathepsin in DCs. However, the deletion of YTHDF1 in classical dendritic cells leads to its inhibition, which significantly enhances the cross-presentation ability of tumor antigen, and makes CD8+T cells highly express PD-L1 and IFN-γ.
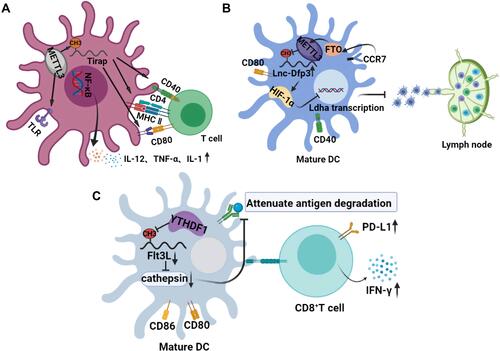
Figure 3 Mechanism of m6A regulation of intestinal mucosal immunity. The folic acid produced by intestinal symbiotic flora such as Lactobacillus and Bifidobacterium, as well as vitamin B2 and B6 in the intestinal tract, participate in SAM synthesis, which are the main raw materials for m6A modification. As an important part of the intestinal mucosal barrier, m6A modification of intracellular XPO1 in IECs further activates the NF-κB pathway and releases inflammatory factors such as IL-8. As an important component of the immune response, the intracellular SOCS m6A modification promotes the activation of the TCR and STAT5 pathway and promotes the proliferation and differentiation of intestinal T cells and the secretion of cytokines such as IL 2/7.
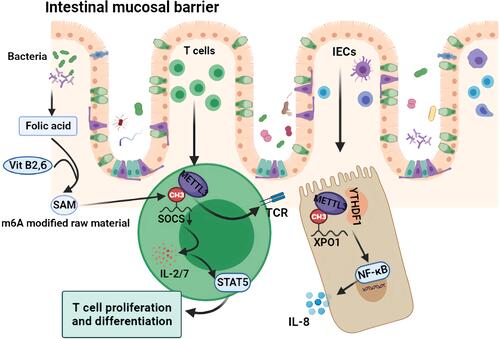
Table 1 Functions of m6A Related Enzymes and Binding Proteins in IBD and CRC Progression
Table 2 Advances in the Mechanism of m6A Modification-Related RNA in the Development of Colitis and CRC
