Figures & data
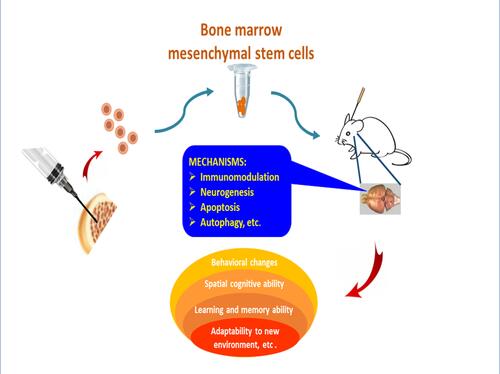
Figure 1 Apoptosis mechanism in Alzheimer’s disease. Extracellular Aβ proteins and inflammatory cytokines (eg, TNF-α, IL-1β) can cause neuronal apoptosis through membrane receptors. The interruption of intracellular homeostasis induces apoptosis via intrinsic pathway as evidenced by oxidative stress and the hyperphosphorylated aggregates of microtubule-associated protein Tau in neurofibrillary tangles. The release of cytochrome c leads to apoptosome formation, which results in caspase activation and subsequent apoptosis. Pro-survival Bcl-2 proteins block the mitochondrial pathway of apoptosis. Endoplasmic reticulum (ER) stress induces apoptosis by initiating calcium-signaling and caspase activation. Inhibitors of apoptosis proteins (IAPs) regulate apoptosis by binding and inhibiting caspases. Mitochondrial Smac/Diablo and Omi/HtrA2 can bind to IAPs to facilitate caspase activation and apoptosis.
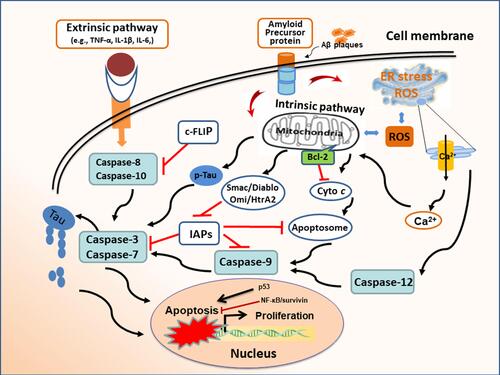
Figure 2 Potential mechanisms of stem cell therapy. The pathological basis of Alzheimer’s disease is neuronal death and the impairment of synaptic transmission, which are concomitant with aberrant Aβ deposits. The transplantation of stem cells derived from bone marrow, adipose tissue, amnion, umbilical cord, or embryonic tissue inhibits neuroinflammation, removes Aβ proteins, and attenuates Tau pathology in the lesion of AD. The comprehensive effect of different mechanisms alleviates neuropathology and improves cognitive deficits in animal models with Alzheimer’s disease.
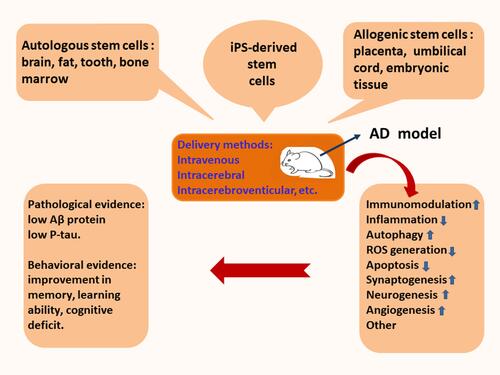
Figure 3 Stem cell therapy induces the inhibition of neuroinflammation and recruitment of peripheral blood monocytes. The transplantation of stem cells leads to the secretion of the autocrine and paracrine factors, which recruits peripheral blood monocytes into the lesion of Alzheimer’s disease. The activated monocytes can accelerate the elimination of aberrant Aβ proteins. Recruited monocytes may facilitate microglial M1/M2 polarization. Neuroinflammation can be inhibited by transplanted stem cells. Immunoregulation participates in functional reconstruction through dynamic remodeling.
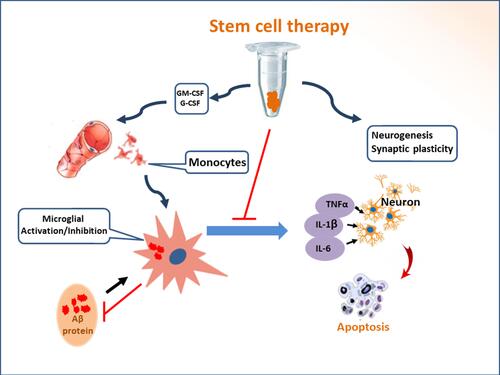
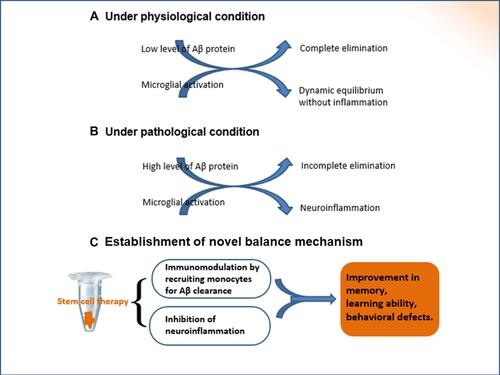
Figure 4 The establishment of new balance mechanism. Under physiological conditions, there is a dynamic equilibrium between the production and elimination of Aβ peptides. If the intrinsic homeostasis is altered, the excessive accumulation of extracellular Aβ proteins results in pathological changes, as shown in the pathogenesis of Alzheimer’s disease. Autocrine and paracrine cytokines are secreted subsequent to the transplantation of BMMSCs, which regulate inflammatory/immune processes. The transplantation of stem cells is key regulator for the establishment of new balance mechanism.