Figures & data
Figure 1 The general process of activation of different inflammasomes. The assembly and function of inflammasomes involves two different steps: signal 1 including various PAMPs and DAMPs turns on the first initiation step, which upregulates the expression levels of inflammasomes components such as pro-IL-1β, pro-IL-18. Then, signal 2 triggers the second activation step, which induces oligomerization of the inflammasome complex including receptor NLRs and ALRs, adaptor ASC, and effector pro-caspase-1, the assembled pro-caspase-1 becomes caspase-1, then cutting pro-IL to activated IL, inducing inflammation response, which plays vital role in inflammation-related diseases including infection-induced injury, autoimmune diseases, autoinflammatory diseases, allergic diseases, GVHD and fibrosis-related diseases.
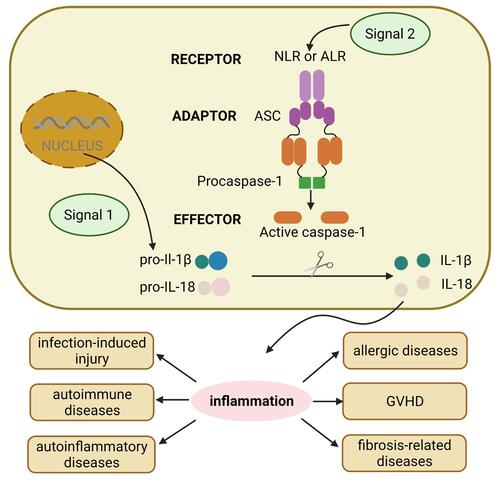
Figure 2 The general regulatory mechanisms of ncRNAs. miRNAs modulate gene expression at the post-transcriptional level either by inhibiting the translation of mRNA or by promoting the degradation of mRNA. lncRNAs can regulate gene expression as signal, guide, decoy and scaffold. circRNAs can exert function at nucleus and cytoplasm. At nucleus, circRNAs can regulate gene transcription and mRNA splicing. At cytoplasm, circRNAs can promote the interaction of proteins, regulate gene translation, be as miRNAs sponging and translate into proteins or peptides.
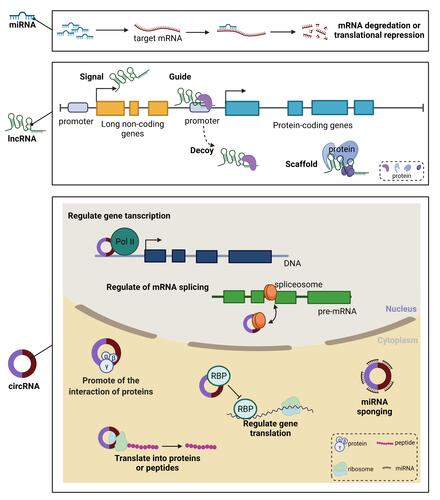
Figure 3 ncRNAs involved in activation of NLRP3 inflammasomes in inflammatory diseases. ncRNAs can regulate both of the two signal for the activation of NLRP3. In the signal 1 step, multiple molecules or inflammatory cytokines activate NF-κB pathway to up-regulate NLRP3, pro-IL-1β, pro-IL-18. Various miRNAs could regulate NF-κB pathway or target components of NF-κB pathway. Several lncRNAs was found to regulate NF-κB pathway as miRNAs’ sponge. The signal 2 is motivated by various endogenous and exogenous stimuli, including ATP, crystals radiation, microbial PAMPs and endogenous DAMPs. These stimuli can induce the activation of diverse molecular and cellular signaling pathways, including K+ efflux, lysosomal damage, ROS generation and mitochondrial dysfunction, which are involved in the activation of the NLRP3 inflammasome. In addition, autophagy has interaction with inflammasomes activation. Various miRNAs, several lncRNAs and circRNAs were found to target components or activation signals of NLRP3 inflammasome pathways.
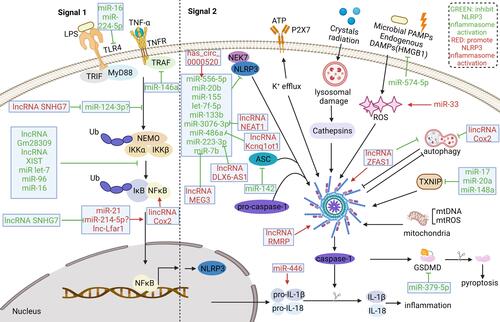
Table 1 The Effect of ncRNAs in Activation of Inflammasomes in Different Inflammatory Diseases
