Figures & data
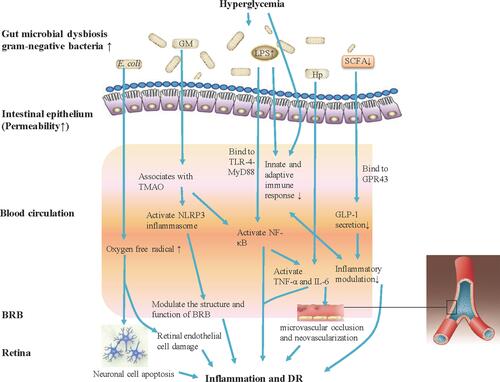
Figure 1 Gut microbiota modulates inflammation and immune mechanism of hyperglycemia-induced DR. Gut microbiota dysbiosis under hyperglycemia with type 2 diabetes (T2DM) causes bacterial translocation and gram-negative bacteria increase allowing accumulated production of LPS into blood circulation. Elevated LPS level activates NF-κB through TLR-4-MyD88, increases the expression of IL-6 and TNF-α, and also can deregulate the innate and adaptive immune response. Increased Hp can induce the expression of IL-6 and TNF-α, and IL-6 can cause damage to vascular endothelial cells. The increasing abundance of E. coli in the microbiome of patients with T2DM will lead to the generation of oxygen free radicals, causing neuronal cell apoptosis and extensive damage to retinal endothelial cells, and promote the development of DR. Gut microbiota associates with the serum levels of TMAO, which promotes vascular inflammation by activating inflammasome, NLRP3, through reactive oxygen species (ROS) signaling pathway. Further, the NLRP3 inflammasome modulates the structure and function of BRB to induce damage of retina. Patients with T2DM commonly have a moderate degree of gut microbial dysbiosis, the abundance of some bacteria that produce butyrate reduction, and impact the levels of SCFA. SCFA production by colonic fermentation, which binds to the GPR43, regulates inflammation and GLP-1 secretion, and further influences the development of DR.